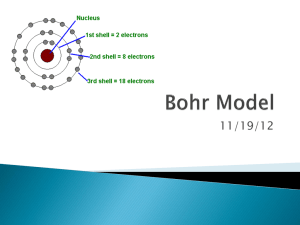
Bohr Model
advertisement
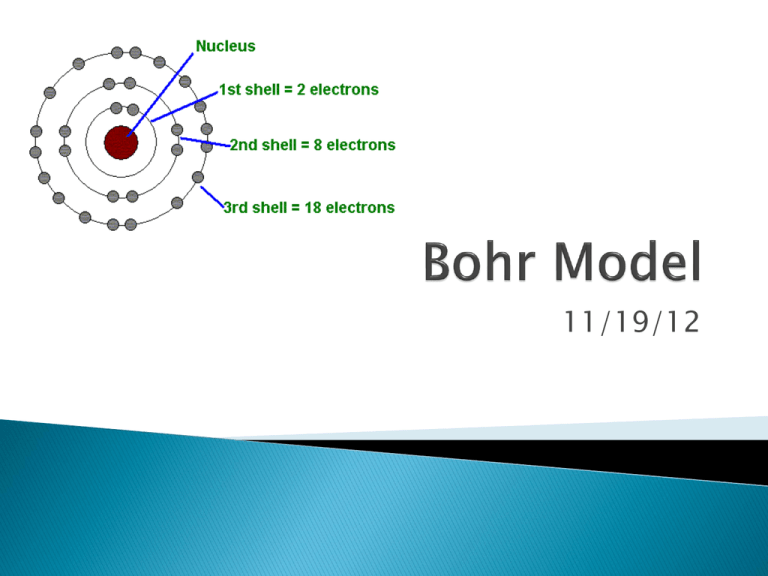
11/19/12 Activity 10 minutes Bell Ringer 5 minutes Announcements 3 minutes Bell Ringer Review 2 minutes Cornell Notes 15 minutes Bohr Model Worksheet Individual Practice 10 minutes Exit Ticket 5 minutes 10 minutes OBJECTIVE S.W.B.A.T describe the placement of subatomic particles and diagram atoms using Bohr Model Bell Ringer Using your knowledge of subatomic particles and atomic structure, draw a Hydrogen atom. **Get your agenda book out after you complete your Bell Ringer** 10/11/2012 3 MINUTES LEFT! 10/11/2012 OBJECTIVE S.W.B.A.T describe the placement of subatomic particles and diagram atoms using Bohr Model Bell Ringer Using your knowledge of subatomic particles and atomic structure, draw a Hydrogen atom. **Get your agenda book out after you complete your Bell Ringer** 10/11/2012 1 MINUTE LEFT!!! 10/11/2012 OBJECTIVE S.W.B.A.T describe the placement of subatomic particles and diagram atoms using Bohr Model Bell Ringer Using your knowledge of subatomic particles and atomic structure, draw a Hydrogen atom. **Get your agenda book out after you complete your Bell Ringer** 10/11/2012 10/11/2012 OBJECTIVE S.W.B.A.T describe the placement of subatomic particles and diagram atoms using Bohr Model Bell Ringer Using your knowledge of subatomic particles and atomic structure, draw a Hydrogen atom. **Get your agenda book out after you complete your Bell Ringer** 10/11/2012 10/11/2012 Monday: Tuesday: Wednesday: Thursday: Friday: Bohr Model Bohr Model with Ions Constructing an Atom Activity Happy Thanksgiving! No School! No School! Be Engaged! (Off Cell Phones, Heads Up, Answering Questions) Mistakes are Welcome! Be Respectful (Speak politely to peers and teachers!) Use Time Preciously! (Be Efficient, Keep Up) Activity 10 minutes Bell Ringer 5 minutes Announcements 3 minutes Bell Ringer Review 2 minutes Cornell Notes 15 minutes Bohr Model Worksheet Individual Practice 10 minutes Exit Ticket 5 minutes 10 minutes ANNOUNCEMENTS Objective: QUIZZES MUST BE FINISHED OR MADE UP BY TUESDAY AFTER SCHOOL!! IF I DON’T HAVE A SCIENCE FAIR IDEA BY WEDNESDAY, I WILL ASSIGN YOU A PROJECT S.W.B.A.T demonstrate mastery (80%) on todays quiz!! Homework: BATHROOM PASS POLICY & DEMERIT SYSTEM WILL BE STRICT THIS QUARTER ALL HOMEWORK MUST BE WRITTEN IN AGENDA BOOKS. MAKE SURE YOU HAVE YOURS EVERY DAY! BOHR MODEL PROBLEM SET LAST WEEKS ASSIGNMENTS EXTRA CREDIT WORKSHEET 10/11/2012 Activity 10 minutes Bell Ringer 5 minutes Announcements 3 minutes Bell Ringer Review 2 minutes Cornell Notes 15 minutes Bohr Model Worksheet Individual Practice 10 minutes Exit Ticket 5 minutes 10 minutes OBJECTIVE S.W.B.A.T describe the placement of subatomic particles and diagram atoms using Bohr Model Bell Ringer Using your knowledge of subatomic particles and atomic structure, draw a Hydrogen atom. **Get your agenda book out after you complete your Bell Ringer** 10/11/2012 Activity 10 minutes Bell Ringer 5 minutes Announcements 3 minutes Bell Ringer Review 2 minutes Cornell Notes 15 minutes Bohr Model Worksheet Individual Practice 10 minutes Exit Ticket 5 minutes 10 minutes Essential Questions: What does an atoms structure look like? How are electrons configured in atoms? What are subatomic particles? How do we determine the number of Subatomic Particles in an atom? The parts that make up an atom. ◦ PEN: Protons, Electron, Neutrons Atomic Number= # of protons & electrons (in a neutral atom) Atomic Mass= #protons + # neutrons Mass # Protons What is the Bohr Model? 1 1 Bohr’s Model was used to explain the energy of atoms Only works for Hydrogen, but is good visualization for atomic structure P+ : 1 E-: 1 N0:1-1= 0 nucleus 1p+ e Electron Energy Shell + - How do we make a Bohr Model? 1. 2. Determine # protons & Neutron draw them in inner circle Determine # Electrons place in energy levels as follows 1st energy level= 2e- max 2nd energy level= 8e- max 3rd energy level= 18e- max LET’S PRACTICE WITH NITROGEN! Nitrogen-14 1. Determine # protons & Neutron draw them in inner circle 2. Determine # Electrons place in energy levels as follows 1st energy level= 2e- max 2nd energy level= 8e- max 3rd energy level= 18e- max P+ EN0 What are Valence Electrons? What’s the Octet Rule? Electrons in the outer most shell ◦ Determine elements chemical properties Atoms gain, lose, or share electrons in order to have 8 valence electrons How do valence electrons effect an elements reactivity? Activity 10 minutes Bell Ringer 5 minutes Announcements 3 minutes Bell Ringer Review 2 minutes Cornell Notes 15 minutes Bohr Model Worksheet Individual Practice 10 minutes Exit Ticket 5 minutes 10 minutes 1 person passes out worksheet (10 seconds!!) 1 person reads directions Ms. Durrette solves #1: 5 min Class solves #2: 5 min With Partner solve #3: 5 min Check with Class Individually work on #4 5min Raise your hand SILENTLY with questions When complete raise your hand for Ms. Durrette to collect your classwork and get your exit ticket! How to draw Bohr Models Activity 10 minutes Bell Ringer 5 minutes Announcements 3 minutes Bell Ringer Review 2 minutes Cornell Notes 15 minutes Bohr Model Worksheet Individual Practice 10 minutes Exit Ticket 5 minutes 10 minutes Activity 10 minutes Bell Ringer 5 minutes Announcements 3 minutes Bell Ringer Review 2 minutes Cornell Notes 15 minutes Bohr Model Worksheet Individual Practice 10 minutes Exit Ticket 5 minutes 10 minutes Silently Complete your Exit Ticket/Reflection When finished raise your hand for Ms. Durrette to collect it Get A Head Start On Your Homework! Extra Credit Opportunity: Complete Bohr Model Practice Worksheet