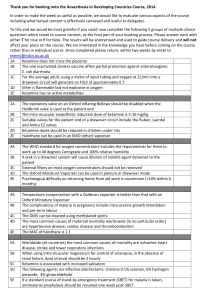
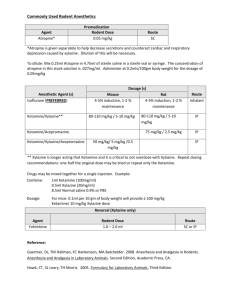
Monitoring PaCCSC studies
advertisement

Monitoring PaCCSC studies Improving quality Flinders University receives funding for PaCCSC from the Australian Government Department of Health and Ageing under the National Palliative Care Program. Acknowledgements Each and every site – Investigators – Site coordinators – Study staff Caroline Litster, Natalie Cutri, Linda Devilee Brief overview PaCCSC has a comprehensive monitoring programme – Unusual for investigator initiated studies – Usual for pharmaceutical sponsored studies – Programme is based on best practice and ICH GCP guidelines • All sites • All participants – Incorporates an element of pragmatism and realistic opportunity Monitoring GCP requirement of sponsors Verifies – rights and well being of participants – protocol has been followed – trial conduct is in compliance with GCP Also a means to verify participant data – Data used in the study analysis is correct and can be verified from other sources – The study results are based on the data from participants – ensures that participant data counts Monitoring Is a detailed review of study documents, protocol implementation and procedures – After study is complete this is the only way to verify compliance Enables assessment of each site and the opportunity to provide support for specific problems Enables training at each site to bring about internal quality improvement Monitoring Assess all site files Review all participant files against source documents Targeted review of critical data elements – – – – – – Eligibility Consent Administration of study medication Review of safety assessments Review of primary outcome assessments Reasons for cessation What worked well Early monitoring included a variety of strategies – Aimed at simply getting through the numbers of files required – Also to increase learning amongst sites and to increase the capacity of all in a period of learning and expansion – Fostered mentoring between recruitment sites and with PaCCSC Ketamine study Monitoring included taking a site coordinator to monitor another site along side the PaCCSC monitor Rotated at least once On site training Ketamine study Enabled each site coordinator to see how another site organised their files, how they prepared for monitoring, and what problems arose Site coordinators could discuss recruitment, filing, organisation and staffing issues Good feedback, and some sites implemented some of the tips seen at the other site Ketamine study Did this process bring about improvements in basic monitoring outcomes for this or future studies The main problems for this study – First study and everyone was learning – Changes in HREC organisation occurred Octreotide study Impending study closure initiated a different monitoring strategy – All study nurses joined a teleconference to review and discuss the specific monitoring needs for this study – Details of monitoring process, paperwork and assessment was discussed – Each study nurse then monitored another site alongside a PaCCSC monitor Octreotide study Study nurses could see for themselves what was monitored, how files at other sites looked, and what problems were found Provided an opportunity to prepare their own files for subsequent monitoring. Octreotide study Did this process bring about improvements in basic monitoring outcomes for this or future studies The main problems for this study – Study was nearing completion and findings could not be implemented at sites for subsequent participants Risperidone study Specific issues related to consent and medication dosing Monitoring has been later than other studies so lessons learned from earlier should be seen here What didn’t work well Organisational factors – Very difficult to arrange dates and sites availability • PaCCSC monitors • Site to be monitored • Site providing support monitor Personnel factors – Much time taken up with training on site, slows down the process – In some cases, networking, discussion and learning resulted in slow file review. Results Did any of these activities result in improvement in monitoring outcomes at sites? Three studies were examined – Ketamine, inpatient study, complete – Octreotide, inpatient study, complete – Risperidone, inpatient study, almost complete General methods Reviewed all the corrective action sheets – Listed the number of actions for each participant at each visit, along with date of randomisation – Listed the number of actions related to general site investigator files – Noted the dates of any training (during ketamine, octreotide, or due to previous monitoring) – Determined the average number of errors for before training and after training. Results Only 1 participant prior to training Ketamine study Only 1 participant after training 4.5 4.0 3.5 3.0 2.5 2.0 1.5 1.0 0.5 0.0 A B C D Average errors prior to training E F G H Average errors post any training I Results Octreotide study 7.00 6.00 Only 1 participant prior to training Did not have training 5.00 4.00 3.00 2.00 1.00 0.00 A B C D E F Average errors prior to training G H I J K Average errors post any training L Results Risperidone study 4.5 No errors in early monitoring 4.0 Only 1 participant after training 3.5 3.0 2.5 2.0 1.5 1.0 0.5 0.0 A B C D E Average errors prior to training F G H I Average errors post any training J Next time Reinforce that monitoring is a QA activity – Results from one visit should be corrected: • For the reviewed file • For next files and future files • For monitored study and other studies Recognise that sites value visits to other sites – to compare notes and see what they are doing – How can we continue to learn and share experiences Next time There are common errors across all studies, and errors specific to a particular study – We need to identify the common errors and determine ways to improve • Consent – Signing, filing, documentation • Documentation • Completion of the CRFs – Answer every question • Ensuring that source documents are clear and available Next time Each site needs to discuss the monitoring outcomes between themselves – Look at the corrective actions – How could this have been avoided – What can be improved for next time and not repeat the same errors What can PaCCSC do to assist?