Future Funding* in Psychology - Office of Sponsored Programs
advertisement

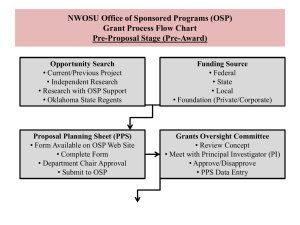
The nuts ‘n bolts of submitting a grant proposal… Trish Lowney, Ph.D. Exe Dir, Office of Sponsored Programs osp.syr.edu | plowney@syr.edu | 113 Bowne Hall Topics Why pursue external funding How to find likely sponsors The Application Process Peer/Merit Review Characteristics of a compelling application Other important matters Key Terms Why pursue external funding Need more resources than have in hand to conduct or disseminate research As a grad student – ◦ Establish track record of success As a post doc ◦ Enables pursuit of *your* interests ◦ Establish or build up track record As a faculty member ◦ Increase amount and pace of work can do ◦ Train next generation of researchers / investigators ◦ Tenure / promotion Proposal development lifecycle Idea Identify Sponsor Develop proposal Internal Feedback OSP Submits Wait 6-9 months Obtain all application materials Internal Approvals Complete application – correct format Award Do good & important Work How to find likely sponsors Ask your advisor / colleagues who’ve come before you Check out the acknowledgements section of your literature Search Community of Science, Illinois Research Information Service ◦ http://osp.syr.edu/Finding%20Funding/Search% 20Engines/subscription-databases.html Then – investigate promising leads…. Investigating promising leads…. Confirm that your interests / needs align with sponsor’s ◦ How? Review sponsor’s mission, current interest, topics.. Review open FOAs Review recent awards ID who will shepherd at sponsor (program officer - PO) ◦ CONTACT PO well in advance of deadline ??s - Is program, mechanism appropriate for proposed project?? Send ‘concept paper’ (see OSP website) What are common proposal weaknesses GOAL - Develop a relationship with this person… Promising leads…. Open FOAs – Grants.gov – the central portal for federal financial assistance Agency solicitations NIH – The NIH Guide: http://grants.nih.gov/grants/guide/index.html NSF – Find Funding: http://www.nsf.gov/funding/ USED IES – Funding Opportunities: http://ies.ed.gov/funding/ Sign up for electronic notification… Promising leads…. Recent awards NIH http://projectreporter.nih.gov/reporter.cfm Includes study section/review panel group Review who members are NSF http://www.nsf.gov/awardsearch/ USED IES http://ies.ed.gov/funding/grantsearch/index.asp Get copies of funded applications… Awarded folks might be future collaborators or colleagues! Sample FOA & solicitation FOA ◦ http://www.grants.gov/search/search.do;jsessionid=3rhDM T8T98ZcFXGjCGG1MMqmdTBpBHflcWvDznWgTvL5Fy GW2p9Y!240750350?oppId=44094&mode=VIEW Instructions Application (pdf ‘envelope’ decorated with attachments) Linked Agency solicitation ◦ http://grants.nih.gov/grants/guide/pa-files/PA-08-169.html Agency /program-specific application instructions FOA + solicitation + application instructions together = information you need to apply Instructions NIH – SF424 (R&R) ◦ http://grants.nih.gov/grants/funding/424/index.htm NSF – the Grant Proposal Guide for FASTLANE ◦ http://www.nsf.gov/pubs/policydocs/pappguide/nsf 11001/gpg_index.jsp USED IES ◦ Not quite generic (varies with CFDA #) ◦ http://ies.ed.gov/funding/ncer_rfas/readwrite.asp Instruction aids.. Does OSP have summaries or checklists for sponsor’s instructions? ◦ Yes – NIH, NSF ◦ If not – ask if can be developed ◦ Use of these summaries does not relieve you of obligation to know all information in application package. Proposal development lifecycle Idea Identify Sponsor Develop proposal Internal Feedback OSP Submits Wait 6-9 months Obtain all application materials Internal Approvals Complete application – correct format Award Do good & important Work The Application Process READ FOA & instructions ◦ This will take healthy chunk of time Mechanics of submission Internal routing & review process Application Instructions – common components Summary (NIH - narrative; other ‘labels’) Research Plan / Narrative / Description Bibliography, references cited Biographical sketches Current & Pending support (NIH – JIT) Budget & justification Facilities, Resources, Equipment Other stuff required or allowed ◦ Follow instructions exactly – this is not time to be creative. Questions? Ask OSP or PO. ◦ Let your narrative convey your enthusiasm, creativity, importance and innovation of your work Application components Research Plan / Description ◦ What you are going to do and how you will do it (among other things) Budget ◦ How much $$ you need ◦ Budget is the financial expression of work plan Understand sponsor’s award limits ◦ NIH – direct costs ◦ NSF, IES – total costs Sponsor award amounts Direct costs, e.g., ◦ R03 - $50k direct costs /yr for 2 years ◦ R21 – max of $250k direct costs for 2 yrs NIH then add IDC to this amount for total award Total costs ◦ Direct + indirect costs ◦ $200k total costs = $137k direct costs for research POINT: Funds available dictate amount of work can do.. Mechanics of Submission Grants.gov (NIH, (PHS), IES, others) DO NOT REGISTER – unless award to individual DO download the ‘application package’ Sign up for ‘alerts’ for solicitation – things change and it’s your obligation to be aware of latest & greatest OSP submits as authorized institutional rep FASTLANE (NSF) Ask OSP to create account – name, degree and year issued Other processes ProposalCentral? Paper? Specific website? Work with OSP. Internal Routing and Review Internal Routing and Review form ◦ osp.syr.edu ◦ Why do we care? We are certifying to the accuracy, completeness, truthfulness of all materials, assuring compliance with all applicable federal regulations, and agreeing to comply with all applicable terms and conditions for award, among other things. We need the fully signed IRR to do this (you do this too on IRR) OSP service guidelines ◦ http://osp.syr.edu/About%20Sponsored%20Programs/ OSP%20Service%20Guidelines/OSP%20Service%20G uidelines.html Proposal development – Plan on being finished 2 weeks before deadline Work backwards from THAT internal deadline Do you need materials from others? Collaborators, advisors, mentors, references? Inform asap, do as much prep work for them a possible OSP will not submit application that names folks from whom we don’t have proper written permission Non-SU individuals: letter of collaboration Non-SU organizations: letter of commitment/consortium agreement New collaboration form coming to help with FFATA requirements Give yourself plenty of time Have others read draft narratives (including Program Officer if you’ve budgeted time) Your advisor and others need to ‘sign off’ on IRR Peer/Merit Review Understand how your application will be reviewed ◦ What’s the process used ◦ Who are the reviewers Is there anything unusual about your application what requires special consideration? ◦ Suggest ad hoc reviews ◦ Select appropriate review panel (NIH CSR) Application must respond to review criteria Characteristics of a compelling application – clearly communicates Great / Important idea! Best approaches to take ◦ Rationale for choices ◦ Alternatives presented ◦ Speed bumps identified Qualified and capable applicant ◦ All expertise needed for success is available ◦ All resources needed for success are available Characteristics of a compelling application “SO WHAT” conveyed… ◦ Significance of idea Context presented and consequences / impact of outcomes achieved ◦ Significance of each aim ◦ Analysis and interpretation of results (expected & unexpected) ◦ Next steps presented Characteristics…. Exciting, informative, importance / significance crystal clear Easy to read Avoid jargon (or clearly define) Remember the reviewer ◦ White space ◦ Judicious use of cartoons, illustrations, tables etc (a picture is worth a thousand words) Follow instructions – ◦ Formatting errors return without review Other important matters Human Subjects Animal Subjects Financial Conflicts of Interest Environmental Health and Safety Summary Understand process Plan ahead (time management) Grant development – not to be done in isolation ◦ Ask for input, help early & often Every institution has an OSP (may have different names) – we’re available to help. Funding opps for grad students.. F31- Pre-doctoral fellowships ◦ http://grants.nih.gov/training/F_files_nrsa.htm F32 – Postdoctoral fellowships ◦ http://grants.nih.gov/grants/guide/pa-files/PA-10110.html Pathways to independence ◦ http://grants2.nih.gov/grants/guide/pa-files/PA-10063.html NSF- SBE Doctoral Dissertation Improvement Grants ◦ http://www.nsf.gov/funding/pgm_summ.jsp?pims_i d=13453&org=NSF&sel_org=NSF&from=fund Key Terms Funding Opportunity Announcement (FOA) ◦ How the federal gov’t communicates to public its interest in supporting a program ordinarily through grant or cooperative agreement HOW: Find Grants.gov and agency sites NIH – Types of FOAs: Program announcement (PA (and PAR / PAS)), Request for Applications (RFA) NSF - Types of FOAs: Dear Colleague Letter, Program Description, Program Announcement, Program Solicitation Financial Assistance ◦ Awards that transfer money, property, or services to a recipient so that it can accomplish a public purpose. Has a catalogue of federal domestic assistance number (CFDA no) Key Terms Grant ◦ Legal instrument for a type of financial assistance – award to institution or individual; ordinarily incorporates provisions from OMB circulars (Office of Mngt & Budget) – A-110, A-21, A-113 Cooperative Agreement ◦ Variation on grant award; has significant involvement /engagement / oversight of agency program officer Procurement ◦ Awards that transfer money to a recipient so that it can address a need or purpose for the benefit the agency. – Agency presents specifications, deliverables etc Key Terms Contract ◦ Legal instrument for procurement; provisions governed by Federal Acquisition Regulations (FAR) Sponsored Program ◦ At SU – any activity supported by a third party through any legal instrument with SU with period of performance. Managed by Office of Sponsored Programs Program officer (or manager, director) ◦ Individual at agency / sponsor who is responsible for overseeing technical aspects of award. Ordinarily is not authorized to obligate agency or funds on behalf of sponsor. Key Terms Grant or contract officer ◦ Individual at agency / sponsor who is responsible for administrative/contractual aspects of award. Is authorized to obligate agency or funds on behalf of sponsor. Principal investigator (or Project Director) ◦ Individual at SU who is responsible for overseeing technical aspects of award. Is not authorized to obligate SU resources. Authorized Institutional Official (Representative) Individual at SU who is responsible for administrative/contractual aspects of award. OSP Research Administrators: authorized to obligate agency or funds on behalf of sponsor. OSP Exe Director: authorized to execute contracts up to $500,000