Feasibility and Reliability of Telemedicine Administration of the Unified Batten Disease Rating Scale:
A Powerful Tool for Rare Disease Clinical Research
Jennifer Cialone, Erika F. Augustine, Nicole Newhouse, Amy Vierhile, Frederick J. Marshall, and Jonathan W. Mink
University of Rochester School of Medicine, Rochester, NY
Introduction
The Neuronal Ceroid Lipofusinoses (NCLs) are a group of rare diseases that affect
1:12,500 children in the United States. The most common NCL is Juvenile Neuronal
Ceroid Lipofuscionosis (JNCL; CLN3 disease; Batten disease),1 an autosomal
recessive neurodegenerative disease of childhood characterized by vision loss,
seizures, dementia, behavioral difficulties, and motor impairment. Symptoms typically
begin around age 5 years; progression results in severe disability then death in the
late 2nd or 3rd decade of life.2-4 To quantify disease progression, we developed a valid
and reliable clinical rating instrument, the Unified Batten Disease Rating Scale
(UBDRS).5 The UBDRS also has potential to be a useful outcome measure for clinical
trials. In its current form, the UBDRS requires in-person evaluation, often requiring
subjects to travel long distances to participate in research studies.
Objective
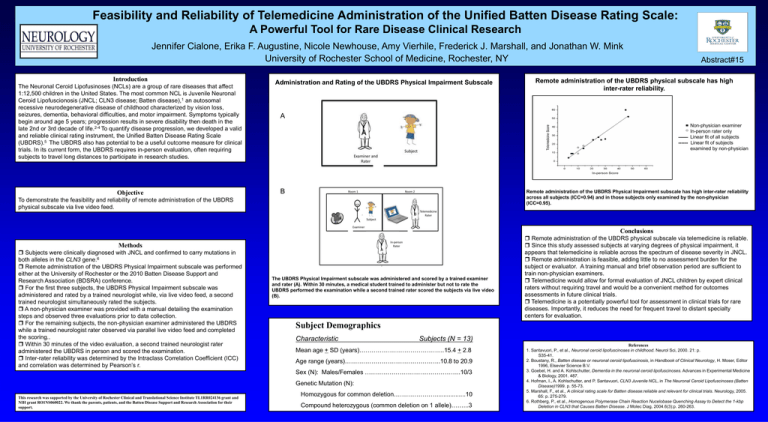
Administration and Rating of the UBDRS Physical Impairment Subscale
Abstract#15
Remote administration of the UBDRS physical subscale has high
inter-rater reliability.
A
Non-physician examiner
In-person rater only
Linear fit of all subjects
------ Linear fit of subjects
examined by non-physician
B
Remote administration of the UBDRS Physical Impairment subscale has high inter-rater reliability
across all subjects (ICC=0.94) and in those subjects only examined by the non-physician
(ICC=0.95).
To demonstrate the feasibility and reliability of remote administration of the UBDRS
physical subscale via live video feed.
Conclusions
Methods
Subjects were clinically diagnosed with JNCL and confirmed to carry mutations in
both alleles in the CLN3 gene.6
Remote administration of the UBDRS Physical Impairment subscale was performed
either at the University of Rochester or the 2010 Batten Disease Support and
Research Association (BDSRA) conference.
For the first three subjects, the UBDRS Physical Impairment subscale was
administered and rated by a trained neurologist while, via live video feed, a second
trained neurologist simultaneously rated the subjects.
A non-physician examiner was provided with a manual detailing the examination
steps and observed three evaluations prior to data collection.
For the remaining subjects, the non-physician examiner administered the UBDRS
while a trained neurologist rater observed via parallel live video feed and completed
the scoring..
Within 30 minutes of the video evaluation, a second trained neurologist rater
administered the UBDRS in person and scored the examination.
Inter-rater reliability was determined by the Intraclass Correlation Coefficient (ICC)
and correlation was determined by Pearson’s r.
The UBDRS Physical Impairment subscale was administered and scored by a trained examiner
and rater (A). Within 30 minutes, a medical student trained to administer but not to rate the
UBDRS performed the examination while a second trained rater scored the subjects via live video
(B).
Subject Demographics
Characteristic
Subjects (N = 13)
Mean age + SD (years)…………………………………...15.4 + 2.8
Age range (years)………………………………………..10.8 to 20.9
Sex (N): Males/Females ………………………………………..10/3
Genetic Mutation (N):
This research was supported by the University of Rochester Clinical and Translational Science Institute TL1RR024136 grant and
NIH grant RO1NS060022. We thank the parents, patients, and the Batten Disease Support and Research Association for their
support.
Remote administration of the UBDRS physical subscale via telemedicine is reliable.
Since this study assessed subjects at varying degrees of physical impairment, it
appears that telemedicine is reliable across the spectrum of disease severity in JNCL.
Remote administration is feasible, adding little to no assessment burden for the
subject or evaluator. A training manual and brief observation period are sufficient to
train non-physician examiners.
Telemedicine would allow for formal evaluation of JNCL children by expert clinical
raters without requiring travel and would be a convenient method for outcomes
assessments in future clinical trials.
Telemedicine is a potentially powerful tool for assessment in clinical trials for rare
diseases. Importantly, it reduces the need for frequent travel to distant specialty
centers for evaluation.
Homozygous for common deletion………………….................10
Compound heterozygous (common deletion on 1 allele)……...3
References
1. Santavuori, P., et al., Neuronal ceroid lipofuscinoses in childhood. Neurol Sci, 2000. 21: p.
S35-41.
2. Boustany, R., Batten disesae or neuronal ceroid lipofuscinosis, in Handbook of Clinical Neurology, H. Moser, Editor
1996, Elsevier Science B.V.
3. Goebel, H. and A. Kohlschutter, Dementia in the neuronal ceroid lipofuscinoses. Advances in Experimental Medicine
& Biology, 2001. 487.
4. Hofman, I., A. Kohlschutter, and P. Santavuori, CLN3 Juvenile NCL, in The Neuronal Ceroid Lipofuscinoses (Batten
Disease)1999. p. 55-73.
5. Marshall, F., et al., A clinical rating scale for Batten disease:reliable and relevant for clinical trials. Neurology, 2005.
65: p. 275-279.
6. Rothberg, P., et al., Homogenous Polymerase Chain Reaction Nucelobase Quenching Assay to Detect the 1-kbp
Deletion in CLN3 that Causes Batten Disease. J Molec Diag, 2004.6(3):p. 260-263.












