17.3
Heat in Changes of State
>
Heats of Vaporization and
Condensation
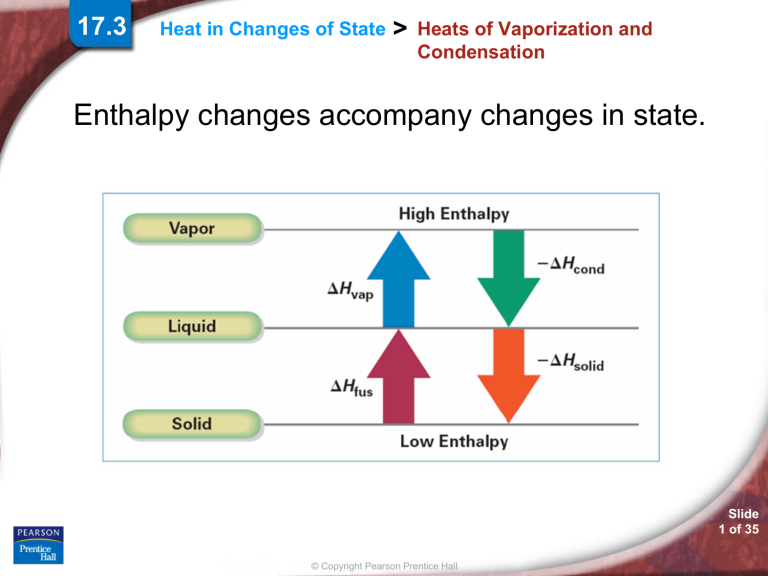
Enthalpy changes accompany changes in state.
Slide
1 of 35
© Copyright Pearson Prentice Hall
17.3
Heat in Changes of State
>
Heats of Fusion and Solidification
The molar heat of fusion (∆Hfus) is the heat
absorbed by one mole of a solid substance as it
melts to a liquid at a constant temperature.
The molar heat of solidification (∆Hsolid) is the
heat lost when one mole of a liquid solidifies at a
constant temperature.
Slide
2 of 35
© Copyright Pearson Prentice Hall
17.3
Heat in Changes of State
>
Heats of Fusion and Solidification
The quantity of heat absorbed by a
melting solid is exactly the same as the
quantity of heat released when the liquid
solidifies; that is, ∆Hfus = –∆Hsolid.
Slide
3 of 35
© Copyright Pearson Prentice Hall
SAMPLE PROBLEM 17.4
Slide
4 of 35
© Copyright Pearson Prentice Hall
SAMPLE PROBLEM 17.4
Slide
5 of 35
© Copyright Pearson Prentice Hall
SAMPLE PROBLEM 17.4
Slide
6 of 35
© Copyright Pearson Prentice Hall
SAMPLE PROBLEM 17.4
Slide
7 of 35
© Copyright Pearson Prentice Hall
Practice Problems for Sample Problem 17.4
Problem Solving 17.21 Solve
Problem 21 with the help of an
interactive guide tutorial.
Slide
8 of 35
© Copyright Pearson Prentice Hall
17.3
Heat in Changes of State
>
Heats of Vaporization and
Condensation
Heats of Vaporization and Condensation
How does the quantity of heat
absorbed by a vaporizing liquid
compare to the quantity of heat
released when the vapor condenses?
Slide
9 of 35
© Copyright Pearson Prentice Hall
17.3
Heat in Changes of State
>
Heats of Vaporization and
Condensation
The amount of heat necessary to vaporize one
mole of a given liquid is called its molar heat of
vaporization (∆Hvap).
The amount of heat released when 1 mol of
vapor condenses at the normal boiling point is
called its molar heat of condensation (∆Hcond).
Slide
10 of 35
© Copyright Pearson Prentice Hall
17.3
Heat in Changes of State
>
Heats of Vaporization and
Condensation
The quantity of heat absorbed by a
vaporizing liquid is exactly the same as
the quantity of heat released when the
vapor condenses; that is, ∆Hvap = –∆Hcond.
Slide
11 of 35
© Copyright Pearson Prentice Hall
17.3
Heat in Changes of State
>
Heats of Vaporization and
Condensation
Slide
12 of 35
© Copyright Pearson Prentice Hall
17.3
Heat in Changes of State
>
Heats of Vaporization and
Condensation
Slide
13 of 35
© Copyright Pearson Prentice Hall
SAMPLE PROBLEM 17.5
Slide
14 of 35
© Copyright Pearson Prentice Hall
SAMPLE PROBLEM 17.5
Slide
15 of 35
© Copyright Pearson Prentice Hall
SAMPLE PROBLEM 17.5
Slide
16 of 35
© Copyright Pearson Prentice Hall
SAMPLE PROBLEM 17.5
Slide
17 of 35
© Copyright Pearson Prentice Hall
Practice Problems for Sample Problem 17.5
Problem-Solving 17.24 Solve
Problem 24 with the help of an
interactive guided tutorial.
Slide
18 of 35
© Copyright Pearson Prentice Hall
17.3
Heat in Changes of State
>
Heat of Solution
Heat of Solution
What thermochemical changes can
occur when a solution forms?
Slide
19 of 35
© Copyright Pearson Prentice Hall
17.3
Heat in Changes of State
>
Heat of Solution
During the formation of a solution, heat
is either released or absorbed.
The enthalpy change caused by dissolution
of one mole of substance is the molar heat
of solution (∆Hsoln).
Slide
20 of 35
© Copyright Pearson Prentice Hall
17.3
Heat in Changes of State
>
Heat of Solution
When ammonium nitrate crystals and water mix
inside the cold pack, heat is absorbed as the
crystals dissolve.
Slide
21 of 35
© Copyright Pearson Prentice Hall
SAMPLE PROBLEM 17.6
Slide
22 of 35
© Copyright Pearson Prentice Hall
SAMPLE PROBLEM 17.6
Slide
23 of 35
© Copyright Pearson Prentice Hall
SAMPLE PROBLEM 17.6
Slide
24 of 35
© Copyright Pearson Prentice Hall
SAMPLE PROBLEM 17.6
Slide
25 of 35
© Copyright Pearson Prentice Hall
Practice Problems for Sample Problem 17.6
Problem-Solving 17.26 Solve
Problem 26 with the help of an
interactive guided tutorial.
Slide
26 of 35
© Copyright Pearson Prentice Hall
17.3 Section Quiz.
Assess students’ understanding
of the concepts in Section 17.3.
Continue to:
-or-
Launch:
Section Quiz
Slide
27 of 35
© Copyright Pearson Prentice Hall
17.3 Section Quiz.
1. The molar heat of condensation of a
substance is the same, in magnitude, as its
molar heat of
a. formation.
b. fusion.
c. solidification.
d. vaporization.
Slide
28 of 35
© Copyright Pearson Prentice Hall
17.3 Section Quiz
2. The heat of condensation of ethanol
(C2H5OH) is 43.5 kJ/mol. As C2H5OH
condenses, the temperature of the
surroundings
a. stays the same.
b. may increase or decrease.
c. increases.
d. decreases.
Slide
29 of 35
© Copyright Pearson Prentice Hall
17.3 Section Quiz
3. Calculate the amount of heat absorbed to
liquefy 15.0 g of methanol (CH3OH) at its
melting point. The molar heat of fusion for
methanol is 3.16 kJ/mol.
a.
1.48 kJ
b.
47.4 kJ
c.
1.52 103 kJ
d.
4.75 kJ
Slide
30 of 35
© Copyright Pearson Prentice Hall
17.3 Section Quiz
4. How much heat (in kJ) is released when 50 g
of NH4NO3(s), 0.510 moles, are dissolved in
water? ssoln = 25.7 kJ/mol
a. 12.85 kJ
b. 13.1 kJ
c. 25.7 kJ
d. 1285 kJ
Slide
31 of 35
© Copyright Pearson Prentice Hall
END OF SHOW






