Graham`s Law
advertisement
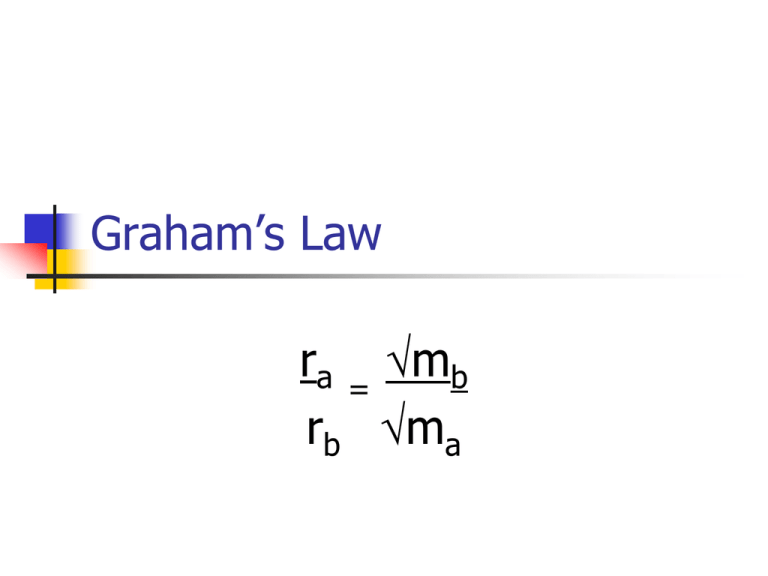
Graham’s Law ra = mb rb ma Graham’s Law ra = rate at which substance a travels rb = rate at which substance b travels ma = mass of substance a mb = mass of substance b Graham’s Law Since rate is distance over time, this equation can also be rearranged as follows: ta = ma tb mb Graham’s Law The practical effect of Graham’s Law is that small molecules travel faster than larger molecules. This is true for both diffusion and effusion. Graham’s Law Diffusion – molecules moving through the air Effusion – molecules moving through very small holes, such as the holes in a balloon Graham’s Law What does this mean about air leaking out of a helium balloon? Graham’s Law Helium, with a mass of 4 amu will leave the balloon faster than nitrogen and oxygen gases with masses of 28 and 32 amu. Graham’s Law Solving problems with Graham’s law is mostly just algebra. Graham’s Law For example: A sample of an unknown gas flows through the wall of a porous cup in 39.9 min. An equal volume of gaseous hydrogen, measured at the same temperature and pressure, flows through in 9.75 min. What is the molar mass of the unknown gas? Graham’s Law We need the equation using time. ta = ma tb mb Graham’s Law What do we know? ta = 39.9 min tb = 9.75 min ma = x amu mb = 2 amu Graham’s Law Plug into the equation: 39.9 = x 9.75 2 Graham’s Law Cross multiply and solve for x 39.9(2) = 9.75(x) 56.43 = 9.75(x) 5.78 = (x) 5.782 = x 33.49 = x











