unit notes power point
advertisement
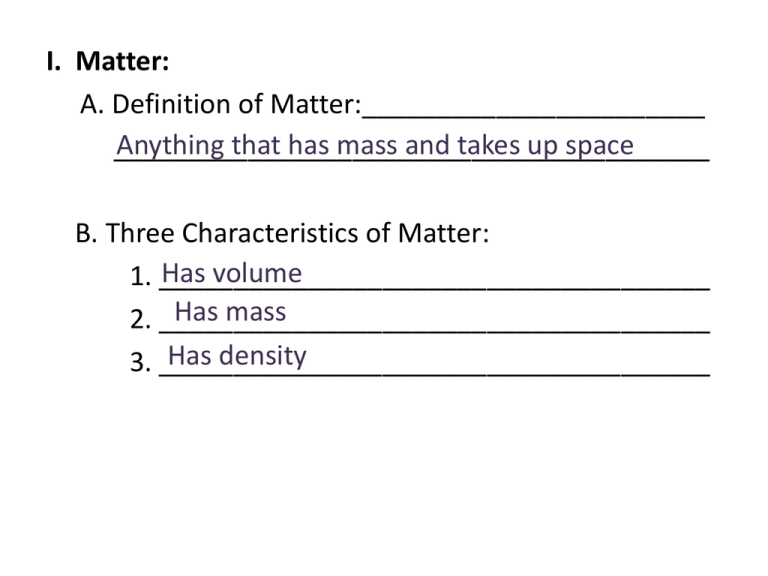
I. Matter: A. Definition of Matter:_______________________ Anything that has mass and takes up space ________________________________________ B. Three Characteristics of Matter: Has volume 1. _____________________________________ Has mass 2. _____________________________________ Has density 3. _____________________________________ II. Density Demonstrations: What is density? Watch the following density demonstrations carefully. After they are all completed, choose one or two that you liked the best and describe (either with words or pictures in the box below to help you remember the concept of DENSITY. A. Based on these above demonstrations and prior knowledge, write a definition of “density” in the space below: B. Pictorial Representation of Density Lower Density Higher Density C. Class Definition of DENSITY: D. Possible Units for Density g/mL = gram per milliliter _________________________________ 3 = gram per cubic centimeter g/cm _________________________________ kg/L = kilogram per liter _________________________________ kg/dm3 = kilogram per cubic decimeter _________________________________ General Theme = “mass”/ “volume” E. The Density of Water 1 g/mL or 1 g/cm3 1. An object will FLOAT in water when its Less than the density of water. density is __________ 2. An object will SINK in water when its density more than the density of water is __________ F. Formula For Density G. Mathematical Density Problems – Class Problem 1. Travel to your lab tables and obtain a diet coke, regular coke double pan balance and a set of weights. 2. Using the double pan balance, determine the mass of both cokes and record below: Mass of Diet Coke: __________________ Mass of Regular Coke:________________ 3. Find the volume (in milliliters) by obtaining an already opened coke container and filling it completely with water. Then transfer all of this liquid to a graduated cylinder and make a reading. Use this amount for the volume of both cans. Volume of Diet Coke:_______________________ Volume of Regular Coke:____________________ 4. Using the density formula, as a team, determine the density of both cokes. Record your answer below. DON’T FORGET UNITS! Density of Diet Coke: (show work) Density of Regular Coke: (show work) 5. Knowing what you do about objects placed in water, as a team, predict what will happen if you place the UNOPENED coke cans in a container of water. Prediction with Diet Coke: (justify your answer) Prediction with Regular Coke: (justify your answer) 6. As a team, travel to the container of water and place both UNOPENED CANS in the water. DO NOT OPEN THE CANS. Make observations as to what happened and compare these observations with your predictions. If they do not match, than determine what you did wrong and FIX IT! Observation with Diet Coke: (relate to prediction) Observation with Regular Coke: (relate to prediction) H. Class project- Determining the volume of a regular shaped object. As a class, we will walk through the process and learn how we can find the volume of a regular shaped object. Using the metric ruler: Using displacement: I. Class project- Determining the volume of an irregular shaped object. As a class, we will walk through the process and learn how we can find the volume of a regular shaped object. Why can’t we use a metric ruler? Because the object does not have measurable sides! Using displacement: Sample Density Problems: 1. The density of a substance that will not mix with water is 0.67 g/ml. When poured into a graduated cylinder of pure water, will the substance be on top or below the water once it settles. 2. The density of corn syrup is 1.38 g/mL. If you have 36 g of corn syrup, what volume (in mL) will it occupy? Density Mass = 26.09 mL 3. What mass will 220 mL of sea water have? (density = 1.03 g/mL) Volume Density = 226.6 g