Chapter 2
advertisement

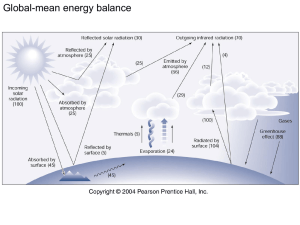
Chapter 2: Warming the Earth and the Atmosphere Temperature scales Different modes of heat transfer Incoming light and seasons Temperature and Heat Transfer Kinetic energy is the energy associated with motion Thus, what is temperature? Temperature is the measure of the average speed of the atoms and molecules The higher the temperature, the faster the molecules are moving Temperature and Density Example Suppose we have a parcel of air A parcel of air is a balloon-like volume of air that does not mix with outside air In the parcel are various molecules moving at a certain speed, and thus the parcel has a certain temperature Now, what happens when we apply heat to the parcel? What happens to the molecules and to the temperature of the entire parcel? Temperature and Density Example According to the definition of temperature, the average speed of the molecules will increase The increase speed of the molecules will create more distance between molecules Thus, the same number of molecules will occupy a greater volume Density is defined as mass/volume (kg/m3) So what happens to the density of the parcel? Temperature and Density Example The opposite happens with cold air If we cool the parcel, the molecules will move slower and crowd together. Thus, the parcel would become more dense So, warm air is less dense than cold air. That is to say, cold air is “heavier” than warm air. More mass in each cubic meter What happens if we continue to cool the parcel? Eventually, it will reach -273°C (-459°F). At this temperature all molecular motion stops. This minimum movement with no thermal motion is called absolute zero What is heat? Heat is the energy transferred between objects due to temperature differences Temperature Scales Absolute zero is the start of the absolute scale, or Kelvin scale of temperature No negative numbers, starts at zero (absolute zero) Introduced by Lord Kelvin 0 K corresponds to –273 on the Celsius scale… Temperature Scales Celsius scale introduced in the eighteenth century 0 degrees is the point at which pure water freezes (freezing point) 100 degrees is point at which pure water boils at sea level One Kelvin degree same as one Celsius degree…so what is the equation? K = °C + 273 Temperature Scales Fahrenheit scale developed in early 1700s 32 degrees is the point at which water freezes (freezing point) 212 degrees is point at which water boils 0 degrees is simply the lowest temperature obtained with a ice salt water Since 1°C is equal to 1.8°F…what is the equation? °C=5/9(°F-32) Fig. 2-2, p. 27 Latent Heat - The Hidden Warmth When water vapor become larger water liquid, this is called change of state or phase change When a substance changes phases, heat is released or taken from the environment (energy is needed) Latent Heat - The Hidden Warmth What happens when you step out of a shower or warm pool? Liquid water on your skin has a certain temperature (remember what temperature is?) The fast moving molecules escape and become vapor. Leaving slower molecules (what has happened to the temperature of the water?) Evaporation is a cooling process!! That is, the energy from the liquid water is “sucked up” into the water vapor. (What about condensation?) Latent heat is heat require to change the phase of a substance. “Latent” because it is “hidden”. Stepped Art Fig. 2-3, p. 28 Conduction Heat transfer between molecules Different substances conduct differently (what are good and bad conductors?) Convection Heat transfer by moving masses Easy for liquids and gases because they can move easily (why does rising air expand and cool, while sinking air compresses and warms?) Advection is the transfer of air prop. to a different location Radiation Radiation is energy transferred by the sun Travels in “waves” and is not released until it is absorbed Travel at the speed of light Measured in micrometers (one millionth of a meter) Photons are energy Fig. 2-7, p. 32 Important Radiation Tips EVERYTHING emits radiation Objects with higher temperatures emit shorter wavelengths Wien’s law (λmax = constant / T). Constant = 2897 μmK What are units of λmax? Objects with higher temperatures emit more total radiation Stefan-Boltzmann law (E=σT4). σ = 5.67 X 10-8 W/m2K4. What are the units of E? Radiation We can only see the visible light portion of the spectrum. Why can we see cool objects then? UV radiation more energetic Infrared radiation less energetic The electromagnetic spectrum tells us from which wavelengths an object is emitting Radiation The sun (10,500°F) emits at a lot of wavelengths (shortwave radiation) A large portion of the emission intensity comes from visible light The surface of earth is cooler (59°F), thus emits almost all infrared radiation (longwave radiation) Radiative equilibrium happens when rate of emission equals rate of absorption Fig. 2-8, p. 34 Fig. 2-9, p. 34 Radiation Earth receives shortwave radiation on half of the surface, but emits longwave radiation from every surface Earth’s radiative equilibrium temperature is 0°F (??) Blackbody – a perfect absorber and emitter (absorbs everything and gets rid of everything) Radiation Why is there a difference between average surface and radiative equilibrium temperatures? The answer is selective absorbers A selective absorber is something that absorbs selective wavelengths and usually emits at similar wavelengths. Name some selective absorbers… Selective Absorbers and the Atmospheric Greenhouse Effect Gases in the atmosphere absorb longwave radiation, but not shortwave radiation well BOTH water vapor and carbon dioxide are strong absorbers of infrared radiation Ozone absorbs shorter wavelengths (in stratosphere) Selective Absorbers and the Atmospheric Greenhouse Effect Atmospheric window is between 8 and 11 μm where atmosphere doesn’t absorb longwave radiation Clouds can close the window because the molecules are larger (more clouds, less radiation leaving) This is why calm, cloudy nights are generally warmer than calm, clear nights Selective Absorbers and the Atmospheric Greenhouse Effect Molecules absorb radiation, causing them to vibrate and bump into neighbors. This increases the kinetic energy, and thus what happens to the atmospheric temperature? Thus, the absorption of longwave radiation by water vapor and carbon dioxide is known as the greenhouse effect, atmospheric greenhouse effect, etc. The Greenhouse Effect Is Essential So, the atmosphere is responsible for warming the surface more Greenhouse effect is essential to keep the surface hospitable Enhancement of the Greenhouse Effect Global warming – increase in atmospheric Carbon Dioxide due to burning of fossil fuels and deforestation Other greenhouse gases on rise as well (CH4, N2O, CFCs) Current predictions increase temperatures 1.4 to 5.8°C How can a small increase in Carbon dioxide increase temperatures? Enhancement of the Greenhouse Effect Positive feedback – a change in a process that will reinforce the process Negative feedback – a change in a process that will weaken the process For instance, rising ocean temperatures…move evaporation, more water vapor (a greenhouse gas), higher temperatures, rising ocean temperatures… Enhancement of the Greenhouse Effect Negative feedback may be…rising ocean temperatures, more evaporation, move water vapor, more clouds, more sun reflected to space, lower temperatures… Warming the Air from Below Radiation from the sun hits the ground Conduction warms the surface and air just above it Convection allows warm air to move upward and cool, maybe condense into clouds Water vapor and Carbon dioxide absorb and emit (in all directions) infrared radiation What Can Happen To Solar Radiation? Scattering – deflection of light in all directions (also known as diffuse light) Sky is blue because Nitrogen scatters BLUE LIGHT! Also responsible For red sunsets What Can Happen To Solar Radiation? What Can Happen To Solar Radiation? What Can Happen To Solar Radiation? Reflected – more light is sent backwards (toward the source) Albedo – percent of radiation returning from a surface (reflectivity) What has a high albedo? Low albedo? What is the Earth’s albedo? What is the Moon’s albedo? The Earth’s Annual Energy Balance The Earth is basically in equilibrium. Thus, it MUST return to space what it receives from the sun. So, we can create a radiation budget The Earth’s surface and atmosphere are also in equilibrium, so we can create a budget there as well Fig. 2-15, p. 41 Fig. 2-16, p. 42 FIGURE 2.16 The earth-atmosphere energy balance. Numbers represent approximations based on surface observations and satellite data. While the actual value of each process may vary by several percent, it is the relative size of the numbers that is important. Stepped Art Fig. 2-16, p. 42 Why the Earth has Seasons Why does Earth have seasons? Closer to sun in January 23.5° tilt of axis Why the Earth has Seasons Seasons have to do with amount of solar energy received Sunlight intensity shows that the equator experiences more intense sunlight Why the Earth has Seasons Longer daylight, more sun reaching the ground, more energy from the sun Sun roams from 23.5°N and 23.5°S (Tropic of Cancer, Tropic of Capricorn) On the Summer Solstice, June 21 (longest “day” of the year in the NH), sun is directly above Tropic of Cancer At Arctic Circle (66.5°N), sun rise on March 20 and does set until September 22 Seasons in the Northern Hemisphere Still cold because sunlight has to pass through a lot of atmosphere that absorbs, scatters, and reflects Why the Earth has Seasons Autumnal Equinox – September 22, when the sun is directly above the equator. NH goes into Fall, SH goes into Spring. Sun sets at North Pole Winter Solstice – December 21, when the sun is directly above 23.5°S. Cold in NH, summer in SH Vernal equinox – March 20, when sun is back over the equator. NH goes into Spring and SH goes into Fall. Sun rises at North Pole Local Seasonal Variations Local Seasonal Variations Slope of hillsides Vegetation differences • Homes can exploit seasonal variations: large windows should face south. • Trees should be planted on west side of house