Energy and Heat Transfer - Atmospheric and Oceanic Sciences
advertisement

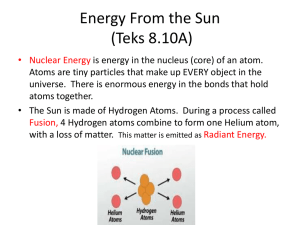
www.spirit-alembic.com Energy and Heat Transfer AOS 101 Discussion Sections 302 and 303 Energy Energy is the capacity to do work Work is done on something when it is either pushed, pulled, or lifted over some distance Kinds of energy Kinetic energy Potential energy KE = ½mv2 PE = mgh Mechanical energy Chemical energy Thermal energy Radiant energy Laws of Thermodynamics 1st Law of Thermodynamics 2nd Law of Thermodynamics Energy cannot be created or destroyed Energy lost during one process must equal the energy gained during another Heat can spontaneously flow from a hotter object to a cooler object, but not the other way around The amount of heat lost by the warm object is equivalent to the heat gained by the cooler object Conservation of Energy Internal energy Amount of heat added Work done Heat Heat is a form of energy and is the total internal energy of a substance Revisiting the 1st law States that heat is really energy in the process of being transferred from a high temperature object to a lower temperature object. Heat transfer changes the internal energy of both systems involved Heat can be transferred by: Conduction Convection Advection Radiation Heat Capacity and Specific Heat Heat capacity of a substance is the ratio of heat absorbed (or released) by that substance to the corresponding temperature rise (or fall) Specific heat The heat capacity of a substance per unit mass Can be thought of a measure of the heat energy needed to heat 1 g of an object by 1ºC Different objects have different specific heat values Specific Heat 1 g of water must absorb about 4 times as much heat as the same quantity of air to raise its temperature by 1º C This is why the water temperature of a lake or ocean stays fairly constant during the day, while the temperature air might change more Because of this, water has a strong effect on weather and climate Substance Specific Heat (J/gK) Water (liquid) 4.183 Ice 2.050 Wood 0.420 Sand 0.835 Air 1.012 Latent Heat Latent heat is the amount of energy released or absorbed by a substance during a phase change FOR WATER: SOLID 334 J/g 2260 J/g released released LIQUID 334 J/g SOLID Lowest energy absorbed GAS 2260 J/g LIQUID LIQUID absorbed GAS Highest energy Orange Example Farmers spray their oranges with water when a frost event is about to occur Why? When the temperature drops below 32oF, liquid water freezes into ice. This liquid to solid phase change causes energy to be released to the fruit. Thus, the temperature of the orange remains warm enough to prevent ruin. Swimming Pool Example Why do you feel cool when you get out of the pool? Drops of liquid water are still on your skin after getting out These drops evaporate into water vapor This liquid to gas phase change causes energy to be absorbed from your skin Cumulus Cloud Example Formation of clouds Clouds form when water vapor condenses into tiny liquid water drops This gas to liquid phase change causes energy to be released to the atmosphere Release of latent heat during cloud formation drives many atmospheric processes Conduction Conduction is the transfer of heat from molecule to molecule within a substance Molecules must be in direct contact with each other Thermal Conductivity Thermal conductivity is the measure of how well a substance can conduct heat Depends on its molecular structure Substance Thermal Conductivity (W/Km) Air 0.024 Soil 0.2 Asphalt 0.75 Glass 1.05 Stainless Steel 16 Copper 401 Silver 429 If it is a bad conductor, it is a good insulator Convection Convection is the transfer of heat by the mass movement of a fluid (such as water and air) in the vertical direction (up and down) physics.arizona.edu Convection Moist Convection As the temperature of an air parcel cools, it may reach a point where it reaches saturation (the air temperature and dew point are the close to the same) Air parcels condense and form a cloud Advection Advection is the transfer of heat in the horizontal direction The wind transfers heat by advection Occurs frequently Why is advection important? Important for the formation of precipitation and fog arcticstudies.pbworks.com Types of Advection Two types Warm air advection (WAA) Wind blows warm air toward a region of colder air Cold air advection (CAA) Wind blows cold air toward a region of warmer air www.aviationweather.ws Radiation Radiation is the travel of energetic particles or waves traveling through space or another kind of medium to heat it up For Example: The suns rays traveling through space and reaching the Earth The warmth from a fire pit Radiation back into space from a warm Earth Black Body A perfect absorber and emitter of radiation Radiation, Convection, and Conduction Solar Radiation The sun’s rays do not hit all areas of the Earth the same Factors that determine the amount of solar radiation hitting the Earth Position on Earth (latitude, longitude, and elevation) Time of day (shown below in UTC) Composition of the atmosphere Amount and thickness of clouds, if any Position of Earth in orbit around the sun (i.e. time of year) Solar Radiation Solar Radiation Equinoxes Where day and night are of equal length Vernal Equinox – March 20 Autumnal Equinox – September 23 Solstices Summer Solstice – June 21 Winter Solstice – December 21 Longest day of the year in the Northern Hemisphere Where the sun is at it’s northernmost point from the equator Shortest day of the year in the Northern Hemisphere Where the sun is at it’s southernmost point from the equator How radiation changes with latitude and date Solar Radiation Budget Energy Budget Radiation All things with a temperature above absolute zero emit radiation Radiation allows heat to be transferred through wave energy These waves are called electromagnetic waves i.e., the sun mainly emits radiative energy in the visible spectrum, and the earth emits radiative energy in the infrared spectrum Wavelengths of the radiation emitted by an object depends on the temperature of that object Shorter wavelengths carry more energy than longer wavelengths Radiation A photon of ultra-violet radiation carries more energy than a photon of infrared radiation The shortest wavelengths in the visible spectrum are purple, and the longest are red Types of Radiation Energy can be: Absorbed Reflected Albedo is the percentage of the light reflected off an object Scattered Increasing the internal energy of the gas molecules Light deflected in all directions: forward, backward or sideways Also called diffused light Transmitted Kirchoff’s Law Good absorbers of a particular wavelength are good emitters at that wavelength and vice versa Our atmosphere has many selective absorbers Carbon dioxide, water vapor, etc… These gases are good at absorbing IR radiation but not solar radiation These gases are called greenhouse gases Due to the fact they help to absorb and reemit IR radiation back toward the Earth’s surface thus keeping us warmer then we would otherwise be More Examples Energy