Chapter 1: Kinetic Particle Theory
advertisement

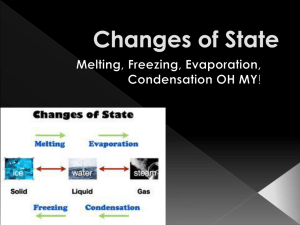
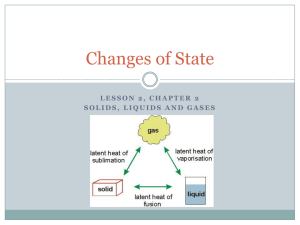
Chapter 1 Kinetic Particle Theory 1.1 States of Matter • Matter can exist as a solid, liquid or a gas • These three forms of matter are known as states of matter • For instance, water(liquid) can exist as ice(solid) or water vapour (gaseous). Changes of State • Melting, freezing, boiling and condensation are example of changes of state 1.2 Kinetic Particle Theory • The kinetic particle theory states that all matter is made up of particles and that these particles are in constant, random motion. Uses of KPT – Describe the states of matter – Explains the differences in particles of solid, liquids, gases – Explains the changes of states. 1.2 Kinetic Particle Theory Solid State • Particles of solid are closely packed in orderly manner • Vibrate about their fixed positions 1.2 Kinetic Particle Theory Why does a solid have a fixed shape and a fixed volume? • Particles of a solid are held together by very strong forces attraction which cannot move freely. • Solid cannot be compressed since its particles are already very close to one another 1.2 Kinetic Particle Theory Liquid State • Particles of a liquid are quite closely packed in disorderly pattern • The particles roll and slide over one another. 1.2 Kinetic Particle Theory Why does a liquid not have a fixed shape? • The force of attraction between the particles are weaker than those in solid. • Particles of a liquid does not have a fixed shape • They can move freely by sliding each other. 1.2 Kinetic Particle Theory Why does a liquid have a fixed volume? • Particles of a liquid are farther away from one another than the particles in a solid. • Particles of a liquid are packed quite closely together. 1.2 Kinetic Particle Theory Gaseous State • Particles of a gas are spread far apart from one another • As the force of attraction between the particles are weak. 1.2 Kinetic Particle Theory Why does a gas not have a fixed shape and a fixed volume? • Particles of a gas have a lot of kinetic energy and are not held in fixed positions. • They can move about rapidly in any direction • Particles can be compressed and move closer to each other. 1.3 Changes of state and the Kinetic Particle Theory Melting When a substance changes from a solid to a liquid, we say that melting takes place. *What happens to particles of a solid that is heated until it melts? As heat energy is supplied, the particles vibrates, until the vibrations of the particles overcome the attractive forces between them. 1.3 Changes of state and the Kinetic Particle Theory Melting Particles begin to break away from their fixed positions. Particles slide over one another it becomes liquid. 1.3 Changes of state and the Kinetic Particle Theory Melting A–B: B A Temperature of the solid increases the unit until it reaches point B (Melting point). Solid begins to melt. 1.3 Changes of state and the Kinetic Particle Theory Melting B-C : B A C Mixtures of solid and liquid exists here. During melting process, temperature of substance remains constant even though heating continues. All heat energy taken in by paritcles is used to overcome force of attraction between particles. 1.3 Changes of state and the Kinetic Particle Theory Melting D B C C-D All solids has melted and temperature of liquid rises as heating continues. A 1.3 Changes of state and the Kinetic Particle Theory Freezing When a substance changes from a liquid to a solid, we say that freezing takes place. *What happens to particles of a liquid that is cooled until it freezes? Particles lose kinetic energy and begin to move more slowly. When the temperature is low enough, some particles start to settle into fixed positions. Finally, all particles settle into fixed positions. Substance now is a solid 1.3 Changes of state and the Kinetic Particle Theory Freezing P P–Q: Q Temperature of liquid drops until it reach point Q, freezing point of napthalene. At point Q, liquid starting freezes. 1.3 Changes of state and the Kinetic Particle Theory Freezing P Q - R: R Q During the freezing process, the temperature of substance remains the same even through cooling continues. 1.3 Changes of state and the Kinetic Particle Theory Freezing R - S: P At point R, substances solidified. Temperature of solid continues to drop as it’s cool. R Q S 1.3 Changes of state and the Kinetic Particle Theory Boiling As the liquid is heated, the particles gain kinetic energy and start to move faster. Eventually, the particles have enough energy to overcome the forces holding them together They spread far apart and move rapidly in all directions. The substance now is a gas. 1.3 Changes of state and the Kinetic Particle Theory Boiling How does the temperature of a liquid change when it boils? When liquid is heated, its temperature increases till its boiling point is reached. Here, it boils & changes into a vapour. The temperature remains constant till all liquid has boiled off 1.3 Changes of state and the Kinetic Particle Theory Evaporation Evaporation occurs because some particles have enough energy to escape as a gas from the surface of the liquid. Liquids that evaporate quickly at room temperature are called volatile liquids. Petrol and perfume are examples of volatile liquids. 1.3 Changes of state and the Kinetic Particle Theory Condensation Heat energy is given out during Condensation As the temperature drops, the gas particles lose energy and move more slower. Eventually, the movement of particles become slow enough for the gas to change into a liquid. 1.3 Changes of state and the Kinetic Particle Theory Sublimation When a substance sublimes, it changes directly from a solid to a gas. Dry Ice, Solid iodine, ammonia chloride sublimes. A substance sublimes because the forces between the particles in liquid state are too weak to remain in the state.