Heat and heat transfer
advertisement

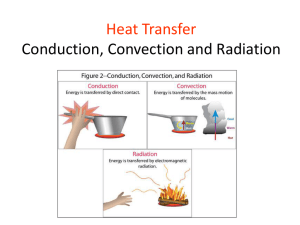
Heat and heat transfer Temperature (T) -Measures how hot something is -Measurement of average (translational) kinetic energy -NOT total kinetic energy (2 different size volumes of water) o o o degrees Celsius or degrees Centigrade or °C Fahrenheit scale Absolute Freezing Kelvin Scale Boiling of zero of water water -273 °C 0 °C 100 °C -460 °F 32 °F 212 °F 0 °K 273 °K 373 °K Heat (Q) -Objects DO NOT have heat - Heat is a transfer of thermal energy - Heat is NOT temperature! They are not interchangeable! - Heat is like work… -Work is the transfer/changing of mechanical energy -Heat flows from hot objects to cold objects - Just like how water flows, it goes from high to low Thermal Equilibrium When two objects are in thermal contact, they will exchange heat from the hot object to the cold object until they reach thermal equilibrium. - Until they are the same temperature A thermometer reaches thermal equilibrium with the material it is in. Thermal Expansion Materials expand when heated and shrink when cooled - there are a few exceptions: Water Transfer of heat When heat is transferred, it is pulled from hotter objects into colder objects. NEVER THE OTHER WAY AROUND! Alton Brown’s Explanation of the transfer of heat and thermoses -Conduction -Convection -Radiation Insulators Vs. Conductors Which of the following are good conductors: Aluminium Glass Copper Iron Polythene Cardboard Nickel Paper Chocolate Steel. • • • • • Good Aluminium Copper Iron Nickel Steel Bad • • • • • Glass Polythene Cardboard Paper Chocolate • Insulator is the special posh sciencey name for a poor conductor. • For the following objects, state whether you would want them to be made out of a good conductor, or a good insulator: • • • • The bottom of a saucepan The handle of a saucepan A duvet/quilt An ice cream tub • Good conductors • Good Insulators • Bottom of a saucepan • Saucepan handle • Duvet/quilt • Ice cream tub Conduction Conduction • Stir your hot soup with a metal spoon • Pretty soon you need a pot holder because the end of the spoon you are holding gets hot • This is heat transfer by conduction • Energy travels up the spoon from the end in the hot soup to the end in your hand Heat transfers along an object metals have high conduction Heating through touch/ contact Conduction Metals have some electrons that are very loosely bound to the atoms in the material These electrons can move easily and can rapidly pick up additional kinetic energy Metals are good conductors Wood and plastic don’t have loosely bound electrons, so they are poor conductors So… When you eat a popsicle, why does the stick feel warmer than the popsicle part if they were both in the freezer together? Convection •Hot air rises because… •It is less dense than cold air •Why? •When something is heated the particles move around more and spread out. •Why does cold gas and liquid fall? •When it cools the particles move around less, move closer together, and therefore become more dense. Convection • A phenomenon in fluids • Instead of having energy moved by successive collisions of electrons, atoms and molecules, the fluid itself is set into motion called a current • These moving fluid currents are convection Convection • When the radiator heats the air, it becomes less dense and rises • Cool air moves in to replace the air that rose • This generates the air flow • So radiators don’t need a fan to stir the air and to distribute heat throughout a room • The rising air cools until its density matches that of the surrounding air Convection • We take advantage of the cooling that occurs during an expansion • We make refrigerators and air conditioners operate by forcing gas under pressure through a small hole and expanding it into an empty space Convection • Explains why breezes come from the ocean in the day and from the land at night Radiation Radiation • Energy carried by electromagnetic waves • Light, microwaves, radio waves, x-rays • Wavelength is related to vibration frequency Radiation • Every object is emitting electromagnetic waves regardless of temperature • Things we can see from their own radiation are very hot to have energy emitted in the visible region of the spectrum • Most things emit primarily in the infrared • Night vision goggles, etc. Radiation • Interior of a car on a sunny day • Sunlight comes in as visible light • Seats and interior are much cooler so they radiate in the infrared instead of visible • Glass in the windows blocks infrared so energy can’t get out • Car interior heats up! Radiation • A good absorber reflects very little energy • Think about dark pavement • A poor absorber reflects a lot of energy • Think about snow that doesn’t melt in sunshine even though 1400 watts/meter2 are hitting it The diagram below represents a saucepan on a hot plate. The saucepan is partially filled with water and the hot plate is turned on. After some time, the air at point X above the pan becomes hot. a. Explain how heat is transferred from the hot plate to the base of the saucepan. b. Explain how heat is transferred from the base of the saucepan to all of the water in the saucepan. c. Explain how heat is transferred from the hot water to the air at point X. The diagram below represents a home heating system. a. Give an example of where heat convection occurs in this system. b. Give an example of where heat radiation occurs in this system. c. What purpose do the ceiling fans serve in helping to heat the home? d. Explain why pipes carrying hot water run along the floors of the rooms instead of the ceilings. Change of Phase When heat is added to, or taken away from a substance, the temperature of the substance will either increase or decrease. However, when a substance changes phase (like when water boils or freezes), heat is need to change the properties of the substance. When this happens, the temperature of the substance will not change, but the substance will change phase. Change of Phase This is known as the latent heat 80 cal/g 540 cal/g Change of Phase •Evaporation from liquid to gas at a surface requires energy (heat) to vaporize a liquid Absorbs heat •Condensation from gas to liquid gives off heat fog and clouds are condensed water vapor •Boiling is a special case of evaporation takes place below the surface 100 °C for water at atmospheric pressure temperature of solid and liquid are the same 540 cal/g to evaporated water Called Heat of vaporization Change of Phase •Freezing from liquid to solid losses energy (heat) to freeze a liquid •Melting –From solid to liquid –Requires heat to melt a solid –Absorbs heat temperature remains constant 80 cal/g of heat lost to freeze water Called Heat of fusion Change of Phase •Sublimation from solid to gas snow can turn to gas directly in winter Think of dry ice •Deposition from gas to solid Water vapor can turn into snow flakes through deposition Latent Heat Latent Heat: The amount of heat required to change the phase of a material. •Dictated by either the Heat of vaporization or the Heat of Fusion •Heat of vaporizations- the heat required to change between liquid and gas (in either direction) •Heat of fusion- the heat required to change between solid and liquid (in either direction) The temperature of an object doesn’t change when it is changing phase, that heat energy actually goes to changing the phase.