Chapter 6 Notes
advertisement

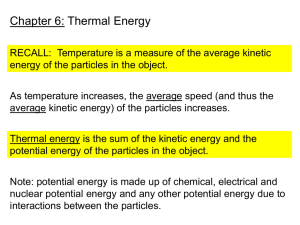
Chapter 6 Thermal Energy 6 – 1 Temperature and Thermal Energy Temperature Do not use “HOT” or “COLD” to describe temperature. Temperature – a measure of the average value of the kinetic energy of the molecules. The Mountain Dew in Mr. Gill’s bottle is not moving, but the atoms that make it up are moving. We are measuring the kinetic energy of the atoms. The greater the kinetic energy, the greater the temperature. Measuring Temperature We cannot measure the kinetic energy of each and every atom in a material. We use a thermometer to measure the expansion and contraction of a material. Used to use mercury, now we use alcohol. Temperature Scales Fahrenheit, Celsius, Kelvin Water : F C K 0C 273K Freezing Point - 32 F Boiling Point - 212 F 100C 373K Converting 9 5 Kelvin = 273 + C Kelvin Scale 0 K is the lowest possible temperature. It is called Absolute Zero. Thermal Expansion When an object warms up : The atoms move faster The atoms tend to spread out This causes the object to expand. When an object cools off : The atoms move slower The atoms tend to move closer together This causes the object to contract ( shrink ) The amount of expansion or contraction depends upon the material and the change in temperature. Liquids tend to expand more than solids Thermal Energy Thermal Energy – the sum of the potential and kinetic energy of the atoms in an object. Kinetic Energy comes from the movement of the atoms. Potential Energy comes from the attractive force between the atoms. Thermal Energy vs. Temperature Thermal Energy and Temperature are different. One does not affect the other. If you have a glass of water and a pitcher of water, both at room temperature, which has more Thermal Energy ? Pitcher because it has more atoms. 6 – 2 Heat Heat Heat is not temperature. Heat – The thermal energy that flows from something with a higher temperature to something with a lower temperature. Place your hand on your desk. Your hand has a higher temperature. Thermal Energy flows from your hand to your desk. If you were to place an ice cube into a glass of water… Does the ice cube cool the drink ? Does the drink warm the ice cube ? By warming the ice cube, the drink loses thermal energy and is cooler. Transferring Thermal Energy 3 Ways Thermal Energy is Transferred : 1. Conduction 2. Convection 3. Radiation Conduction Conduction – the transfer of thermal energy through matter by direct contact of particles. Object # 1 to Object # 2 Throughout an entire object Collisions The particles collide with one another. Momentum is transferred from one particle to the next. Efficiency 1. 2. 3. Solids work best because their particles are packed the closest together. Liquids Gases Conductors The ability to easily transfer thermal energy. Metals Loosely held electrons make metals work well. Silver, Copper, Aluminum, Gold Insulators Do not easily allow the transfer of thermal energy between particles. Wood, Plastic, Glass, Fiberglass Convection Convection – the transfer of thermal energy by the bulk movement of matter. Requires a Fluid Fluid – any material that flows. Liquids and Gases Convection Currents The rotation caused by warmed fluids rising and cooled fluids sinking. Winds ( weather ) Ocean Currents Radiation Radiation – the transfer of thermal energy in the form of waves. Radiant Energy – energy that travels in the form of radiation. Heat Absorption Why is it that the pavement is hotter than grass ? The change in temperature of a material as it absorbs heat depends upon the material it is made of. Specific Heat Specific Heat – the amount of thermal energy needed to raise the temperature of 1 kg of a substance 1 °C. The greater the specific heat, the more energy is needed to change the temperature of a substance. Water has a very high specific heat. Thermal Pollution Thermal Pollution – the increase in the temperature of a body of water caused by adding warmer water. Some electric power plants and factories that use water to cool machinery produce hot water as a by-product. If the hot water is placed back into its source, it will raise the temperature of the water nearby. Effects of Thermal Pollution Higher water temperature requires fish to use more oxygen. Warmer water contains less dissolved oxygen. Fish and other organisms can die. Reducing Thermal Pollution Cooling towers are used to bring water temperatures back down closer to the temperature of the main body of water. 6 – 3 Engines and Refrigerators Heat Engine Heat Engine – A device that converts thermal energy into mechanical energy. Engines in cars, trucks, motorcycles, tractors. Mechanical Energy – The sum of the kinetic and potential energy of an object. Differs from thermal energy because this is the energy of the object, not the energy of the atoms that make it up as in thermal energy. Internal Combustion Engine The fuel is burned on the inside of the engine in a combustion chamber. Most cars have an engine that has 4 or more cylinders. A mixture of fuel and air is injected into the cylinder and ignited by a spark. The burning fuel produces a gas. The expansion of this gas pushes the piston down. Each cylinder contains a piston that moves up and down. The up and down motion of the piston turns a crankshaft that turns the wheels. Heat Mover Heat Mover – A device that moves thermal energy from one location, having a lower temperature, to another location having a higher temperature. A refrigerator, air conditioner, freezer. A refrigerator absorbs thermal energy from food and carries it to outside the refrigerator. Air conditioners work under the same principles as a refrigerator. Thermal energy is absorbed from inside the building and transferred to outside the building. A heat pump can move thermal energy either into or out of a building.