Heat
advertisement

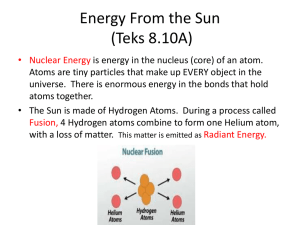
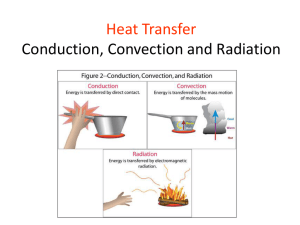
AP Physics Chapter 11 Heat Chapter 11: Heat 11.1 11.2 11.3 11.4 Units of Heat Omitted Omitted Heat Transfer Learning Objectives 1. Mechanical Equivalent of Heat a. Students will understand the “mechanical equivalent of heat” so they can determine how much heat can be produced by the performance of a specified quantity of mechanical work. 2. Heat Transfer and Thermal Expansion a. Students will understand heat transfer and thermal expansion, so they can analyze qualitatively the effects of conduction, radiation, and convection in thermal processes. Homework for Chapter 11 • Read Chapter 11 • HW 11: p.383: 3-7. p.386: 54,55,57-59,61,62. 11.1 Units of Heat Warmup: Meat Platter Physics Warmup #87 Each decade seems to have its own collection of unique kitchen items sold through television and specialty catalog advertising. One such item sold in the ’90s was a “magic” defrosting plate. You simply laid the frozen meat on the plate and in a few minutes the meat was ready for cooking. Manufacturers claimed to have sold several million of these in just one year. ********************************************************************************************* Several science-oriented publications printed articles describing the defrosting plate phenomenon. Although the various brands differed in size and thickness, they all were similar in that they were simply a metal block painted black. Users were instructed to run hot water over the plate after a few minutes to renew its defrosting abilities. Identify which method of heat transfer was being used (conduction, convection, or radiation), and explain how running hot water over the plate renewed its ability to defrost. Answer: Being at a higher temperature, the block transferred heat to the meat by conduction. As the block cooled down, the rater of conduction slowed. Hot water raised the temperature of the block. Warmup: Hot ‘n’ Cold II Physics Warmup #88 Any material that does note conduct heat well is a thermal insulator. Air is a poor conductor of heat and is the main ingredient in many thermal insulation materials. ********************************************************************************************* Explain why Styrofoam cups work equally well at keeping hot drinks hot and cold drinks cold, while still making it possible for you to hold onto the cup. Answer: Styrofoam is mainly air. Heat travels through it poorly; it can’t leave the hot coffee or get to the cold drink. Heat is the net energy transferred from one object to another because of a temperature difference. • Heat flows naturally from a warm body to a cool body in contact until they reach the same temperature, thermal equilibrium. The SI unit of heat is the joule (J). Other units of heat commonly used are the: calorie (cal ) kilocalorie (kcal) Calorie (C) a food Calorie, which is the same as a kcal. Note the capital C for the “large” food calorie. 1 food calorie = 1Cal = 1,000 cal British thermal unit (Btu) 1 Btu = 252 cal a) A kilocalorie raises the temperature of 1 kg of water by 1 C°. b) A calorie raises the temperature of 1 g or water by 1 C°. c) A Btu raises the temperature of 1 lb of water by 1 F°. mechanical equivalent of heat - the relationship between unit of heat and the standard SI unit (J). • The mechanical equivalent of heat, 4.186 joules, is the amount of work equal to the transfer of one calorie of heat. 1 kcal = 4,186 J = 4.186 kJ 1 cal = 4.186 J Example 11.a: A piece of candy supplies 27.5 Calories. If this energy is converted into work for lifting a load, how much mass can be lifted to a height of 5 ft (approx. 1.52 m) with it? 11.4 Heat Transfer Warmup: Hot ‘n’ Cold I Physics Warmup #86 Heat can be transmitted by three different methods. When hot objects touch cooler objects, heat is transmitted by conduction. As a warmer fluid moves into a cooler region, heat is transmitted by convection. The transmission of heat through electromagnetic waves is called radiation. Any material that effectively reduces the transmission of heat is called a thermal insulator. ********************************************************************************************* Thermos bottles have been used for many years to keep coffee hot for many hours. They basically consist of double glass walls, which are coated with a silvercolored coating. The air between the double walls has been pumped out, creating a vacuum. Explain the roles that the silver coating and the vacuum play in making the thermos bottle such an effective thermal insulator. Answer: The silver lining prevents loss due to radiation and the vacuum prevents loss due to conduction. Warmup: The Cooking Glass Physics Warmup #85 Certain materials not only transmit heat well through conduction, they transmit it evenly throughout the material. Unfortunately, regular glass is not one of those materials. It does a good job of transmitting heat, it just doesn’t do it evenly. ********************************************************************************************* Pyrex glass was specially developed to overcome the problem with uneven heating. As a result, it only expands half as much when heated as regular glass. Explain why Pyrex glass is better than regular glass to use when cooking hot dishes. Answer: While the more even heating is somewhat beneficial, the fact that it is far less likely to shatter is the main reason it is better. 11.4 Heat Transfer Examples: Heating water in a beaker over a Bunsen burner Heating of the earth’s atmosphere warming of air in a room Radiation does not require material medium; it can take place through a vacuum. The radiation can also pass through some material media such as water, glass, and air, etc. The solar heat comes to the earth through millions of kilometers of vacuum. The hands at the left are warmed by the convection of rising hot air (and some radiation). The gloved hand at upper right is warmed by conduction. The hands at lower right are warmed by radiation. On Gold Sheet k H= H k L L = rate of heat transfer thermal conductivity (k) – characterizes the heat-conducting ability of a material. • The greater the value of k for a material, the more rapidly it will conduct heat. • The units of k are J/(m·s·C°) or kcal /(m·s·C°). Thermal Conductivities of Some Substances (see also p.374 in text) Thermal conductivity (k) Substance J/(m·s·C°) kcal /(m·s·C°) Aluminum 240 5.73 x 10-2 Copper 390 9.32 x 10-2 Iron and steel 46 1.1 x 10-2 Water 0.57 14 x 10-5 Air 0.024 0.57 x 10-5 Brick 0.71 17 x 10-5 Glass 0.84 20 x 10-5 Styrofoam 0.042 1.0 x 10-5 Vacuum 0 0 Example 11.4: A window glass 0.50 cm thick has dimensions of 2.0 m by 1.0 m. If the outside temperature is -10°C and the inside temperature is 20°C, a) What is the rate of heat conduction through the window? b) How much heat flows through the window in 1.0 hours due to conduction only? Check for Understanding 1. The SI unit of heat energy is the: a) calorie b) kilocalorie c) Btu d) joule Answer: d 2. The warming of the atmosphere involves a) b) c) d) conduction convection radiation all of these Answer: d 3. Water is a very poor heat conductor. Why can it be thoroughly heated relatively quickly? a) b) c) d) conduction convection radiation all of these Answer: b. The warm (less dense) water rises to the top and the cooler (more dense) water sinks to the bottom. 4. A plastic ice tray and a metal ice tray are removed from the same freezer (initial temperature). However, once your hands touch both, the metal one feels cooler. Why? Answer: Metal has a higher heat conductivity so it conducts heat away from your hand faster. 5. You blow on a spoonful of hot soup to cool it. Yet, your breath is warm. How does blowing cause the soup to cool? Answer: This convects the heat from the hot soup to the cooler air. Homework 11 • HW 11: p.383: 3-7. p.386: 54,55,57-59,61,62.