100 km Dz - Atmospheric Chemistry Modeling Group

EPS200: Atmospheric Chemistry
Instructors: Daniel J. Jacob and Steven C. Wofsy
Teaching Fellow: Helen M. Amos
EPS 200 is intended as a “core” graduate course in atmospheric chemistry
• Assumes no prior knowledge of atm chem
• Suitable as “breadth” for students in other fields
• complements other core course EPS208 (Physics of Climate)
• broad survey of field, prepares for + complements more advanced courses:
EPS 236 Environmental Modeling
EPS 238 Spectroscopy and Radiative Transfer of Planetary Atmospheres
ES 267 Aerosol Science and Technology
ES 268 Environmental Chemical Kinetics
Disasters
BIG PROBLEMS IN ATMOSPHERIC CHEMISTRY
Visibility
Ozone layer
Urban smog
Regional smog Climate
Point source
LOCAL
< 100 km
Acid rain
REGIONAL
100-1000 km
Biogeochemical cycles
GLOBAL
> 1000 km
GLOBAL OBSERVING SYSTEM FOR TROPOSPHERIC COMPOSITION
Satellites
Surface networks
Chemical transport models (CTMs)
Aircraft, ships
CTMs solve coupled continuity equations for chemicals on global 3-D Eulerian grid:
Emissions
Transport
Chemistry
Aerosol processes
Deposition
D x ~100 km
D z ~ 1 km
C t i C i
P i
C
L i
C
ATMOSPHERIC STRUCTURE AND TRANSPORT
“SEA LEVEL” PRESSURE MAP (9/2/10, 23Z)
SEALEVEL PRESSURE CAN’T VARY OVER MORE
THAN A NARROW RANGE: 1013 ± 50 hPa
Consider a pressure gradient at sea level operating on an elementary air parcel dxdydz :
P(x) P(x+dx)
Vertical area dydz
Pressure-gradient force
Acceleration
d F
( ( )
(
))
1 dP
dx
For
D
P = 10 hPa over
D x = 100 km,
~ 10 -2 m s -2 a 100 km/h wind in 3 h!
Effect of wind is to transport air to area of lower pressure a dampen
D
P
On mountains, however, the surface pressure is lower, and the pressure-gradient force along the Earth surface is balanced by gravity:
P(z+
D z)
P-gradient gravity a This is why weather maps show “sea level” isobars; a The fictitious “sea-level” pressure at a mountain site assumes an air column to be present between the surface and sea level
P(z)
MASS m a
OF THE ATMOSPHERE
Radius of Earth:
6380 km
Mean pressure at Earth's surface:
984 hPa m a
4
2
R P
Surface g
Total number of moles of air in atmosphere:
N a
m
M a a
20
1.8 10 moles
Mol. wt. of air: 29 g mole -1 = 0.029 kg mole -1
VERTICAL PROFILES OF PRESSURE AND TEMPERATURE
Mean values for 30 o N, March
Stratopause
Tropopause
Barometric law (variation of pressure with altitude)
• Consider elementary slab of atmosphere:
P(z+dz)
P(z)
( )
(
dz )
a gdz unit area
Ideal gas law:
a
PM a
RT
dP
M g
P RT dz
dP
a g dz hydrostatic equation
Assume T = constant, integrate:
( )
P (0) e
with
scale height
H
RT
M g a
7.4 km ( T
250 K)
Barometric law n z a
n a
(0) e
(
H )
e
; (
5km)
2
Application of barometric law: the sea-breeze effect
ATMOSPHERIC TRANSPORT
Forces in the atmosphere:
• Gravity g
• Pressure-gradient
• Coriolis
• Friction
γ c f
2
k v v
γ p sin
1/
P to R of direction of motion (NH) or L (SH)
Equilibrium of forces:
In vertical: barometric law
p
In horizontal: geostrophic flow parallel to isobars
c
In horizontal, near surface: flow tilted to region of low pressure
p
f v
c v
P
P +
D
P
P
P +
D
P
Air converges near the surface in low pressure centers, due to the modification of geostrophic flow under the influence of friction. Air diverges from high pressure centers. At altitude, the flows are reversed: divergence and convergence are associated with lows and highs respectively
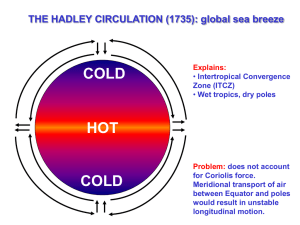
THE HADLEY CIRCULATION (1735): global sea breeze
COLD
Explains:
• Intertropical Convergence
Zone (ITCZ)
• Wet tropics, dry poles
• General direction of winds, easterly in the tropics and westerly at higher latitudes
HOT
COLD
Hadley thought that air parcels would tend to keep a constant angular velocity.
Meridional transport of air between Equator and poles results in strong winds in the longitudinal direction.
…but this does not account for the Coriolis force correctly.
TODAY’S GLOBAL INFRARED CLOUD MAP (intellicast.com) shows Intertropical Convergence Zone (ITCZ) as longitudinal band near Equator
Today
Bright colors indicate high cloud tops (low temperatures)
TROPICAL HADLEY CELL
• Easterly “trade winds” in the tropics at low altitudes
• Subtropical anticyclones at about 30 o latitude
CLIMATOLOGICAL SURFACE WINDS AND PRESSURES
(January)
CLIMATOLOGICAL SURFACE WINDS AND PRESSURES
(July)
500 hPa (~6 km) CLIMATOLOGICAL WINDS IN JANUARY: strong mid-latitude westerlies
500 hPa (~5 km) CLIMATOLOGICAL WINDS IN JULY mid-latitude westerlies are weaker in summer than winter
ZONAL GEOSTROPHIC FLOW AND THERMAL WIND RELATION
gz geopotential height
= latitude a = Earth radius
= angular vel. of Earth
p
1
P
y
1 y a
P u
P +
D
P
c
2
u sin
fu
y f z
*
H ln( / o
) log-P coordinate
H
RT o
Mg
scale height
Geostrophic balance: fu
1 a
Thermal wind relation: f
u
z
*
R
T aH
x
ZONAL WIND: VARIATION WITH ALTITUDE follows thermal wind relation
TIME SCALES FOR HORIZONTAL TRANSPORT
(TROPOSPHERE)
1-2 months
1-2 months
2 weeks
1 year
Illustrates long time scale for interhemispheric exchange
Dust transport over the Pacific, April 21-25, 1998
• What is buoyancy?
R. Husar
TRANSPORT OF ASIAN DUST TO NORTH AMERICA
Clear day April 16, 2001: Asian dust!
Glen
Canyon,
AZ
Mean April 2001
PM concentrations measured by MODIS
GLOBAL TRANSPORT OF CARBON MONOXIDE (CO)
Sources of CO: Incomplete combustion (fossil fuel, biofuel, biomass burning), oxidation of VOCs
Sink of CO: atmospheric oxidation by OH radical (lifetime ~ 2 months)
MOPITT satellite observations of
CO concentrations at
500 hPa (~6 km)
OBSERVATION OF CO FROM AIRS SATELLITE INSTRUMENT
AIRS CO data at 500 hPa (W.W. McMillan)
Averaging kernels for AIRS retrieval
ATMOSPHERIC LAPSE RATE AND STABILITY
“Lapse rate” = dT/dz z stable unstable
Consider an air parcel at z lifted to z+dz and released.
It cools upon lifting (expansion). Assuming lifting to be adiabatic, the cooling follows the adiabatic lapse rate
G
:
G
= 9.8 K km -1
G
dT dz
g
C
p
9.8 K km
-1 z inversion unstable
ATM
(observed)
T
What happens following release depends on the local lapse rate – dT
ATM
/dz:
•
dT
ATM
/dz >
G e upward buoyancy amplifies initial perturbation: atmosphere is unstable
• dT
ATM
/dz =
G e zero buoyancy does not alter perturbation: atmosphere is neutral
• dT
ATM
/dz <
G e downward buoyancy relaxes initial perturbation: atmosphere is stable
• dT
ATM
/dz > 0 (“inversion”): very stable
The stability of the atmosphere against vertical mixing is solely determined by its lapse rate.
WHAT DETERMINES THE LAPSE RATE OF THE
ATMOSPHERE?
• An atmosphere left to evolve adiabatically from an initial state would eventually tend to neutral conditions (dT/dz =
G at equilibrium
• Solar heating of surface and radiative cooling from the atmosphere disrupts that equilibrium and produces an unstable atmosphere: z z z
ATM
G
G
ATM initial final
G
T T T
Initial equilibrium state: dT/dz =
G
Solar heating of surface/radiative cooling of air: unstable atmosphere buoyant motions relax unstable atmosphere back towards – dT/dz =
G
• Fast vertical mixing in an unstable atmosphere maintains the lapse rate to
G.
Observation of dT/dz =
G is sure indicator of an unstable atmosphere.
IN CLOUDY AIR PARCEL, HEAT RELEASE FROM
H
2
O CONDENSATION MODIFIES
G
Wet adiabatic lapse rate
G
W
= 2-7 K km -1 z
T
RH
G
W
100%
“Latent” heat release as H
2
O condenses
G
W
2-7 K km -1
RH > 100%:
Cloud forms
G
9.8 K km -1
G
4
3 cloud
2
1
0
-20 -10 0 10 20 30
Temperature, o C boundary layer
SUBSIDENCE INVERSION typically
2 km altitude
DIURNAL CYCLE OF SURFACE HEATING/COOLING: ventilation of urban pollution z
Subsidence inversion
PBL depth
MIDDAY
1 km
G
Mixing depth
NIGHT
0
MORNING
T NIGHT MORNING AFTERNOON
VERTICAL PROFILE OF TEMPERATURE
Mean values for 30 o N, March
Radiative cooling (ch.7)
- 3 K km -1
2 K km -1
Radiative cooling (ch.7) - 6.5 K km -1
Radiative heating:
O
3
+ h
O + O
2 n e
+ M
O
2
+ O e
O
3
+M heat
Latent heat release
Surface heating
LATITUDINAL STRUCTURE OF TROPOPAUSE REGION
RADIATIVE-CONVECTIVE EQUILIBRIUM ATMOSPHERE
z
BAROCLINIC INSTABILITY q
3 > q
2
> q
1
Buoyant vertical motion
Is possible even when q
0
0 latitude
Dominant mechanism for vertical motion in extratropics
FIRST-ORDER PARAMETERIZATION OF TURBULENT FLUX
• Observed mean turbulent dispersion of pollutants is near-
Gaussian e parameterize it by analogy with molecular diffusion:
Time-averaged envelope z
Near-Gaussian profile
Instantaneous plume
Source
Turbulent flux =
K z n a
C
z
Turbulent diffusion coefficient
<C>
• Typical values of K z
: 10 2 cm 2 s -1 (very stable) to 10 7 cm 2 s -1 (very unstable); mean value for troposphere is ~ 10 5 cm 2 s -1
• Same parameterization (with different K x
, K y
) is also applicable in horizontal direction but is less important (mean winds are stronger)
TYPICAL TIME SCALES FOR VERTICAL MIXING
• Estimate time
D t to travel
D z by turbulent diffusion:
2
with K z
2 K z
2 -1 tropopause
(10 km)
10 years
“planetary 2 km boundary layer”
0 km
5 km
1 week
1 month
1 day