TOPIC: Heat AIM: How is heat measured?
advertisement
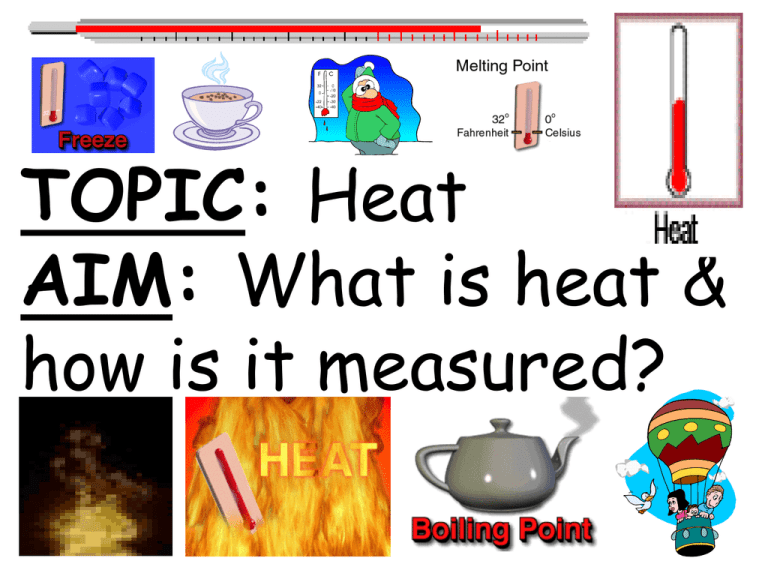
TOPIC: Heat AIM: What is heat & how is it measured? • What you will learn: 1.Define temp. 2.Explain how thermal energy and temp are related. 3.Calculate the change in thermal energy of an object due to temp change. • Why is it important: Cars, buses, trucks and airplanes could not operate without thermal energy. What is kinetic energy? •Review vocab: •Kinetic energy: energy an object has due to motion • New Vocab • Kinetic theory • Kelvin • Temp • Thermal energy • Heat • Specific heat Kinetic Theory of Matter Matter is made of molecules that are always in motion which collide & transfer their Kinetic Energy • Molecules are always moving which produces heat •The more heat that is contained the faster the molecules move What are molecules? Molecules • Tiny particles that make up matter • Example: 1 molecule of water = H 2O 5 molecules of water • Molecules moving (have KE) What is • Energy caused by internal motion of heat? molecules • Energy moves from warm cooler region Temperature •Measure of how hot or cold something is •Measure of average KE of molecules If heat is added to an object the faster molecules move ________, which increases the Kinetic Energy, _________ which causes the temperature to _________. increase • Low temp = slower moving • High temp = fast moving The average kinetic energy of the particles in the soup is greater than the average kinetic energy of the particles in the ice cream. 70°F 70°F The pail of water has fewer molecules in it than the ocean does. Even though they may be the same temperature, the pail of water has less heat than the ocean. Thermometer • For measuring temperature • Tube filled with a fluid such as mercury • Heated molecules move faster and far apart • Mercury expands & rises up tube • Cooled molecules slow & move closer together • Mercury contracts and drops Heated Liquid level rises Cooled Liquid level drops •Non-metric temp scale Fahrenheit Scale °F • Water freezes = 32°F • Water boils = 212°F • Human body temp = 98.6°F Celsius Scale °C •Metric scale • Water freezes = 0°C • Water boils = 100°C • Human body temp = 37°C Kelvin •Metric scale scale •Units = Kelvins (K) • Absolute Zero = 0K • (coldest temp = when molecules stop moving) Conversions °C K •Celsius + 273K K °C •Kelvin - 273 Example: 56°C = ? K •56 + 273 = •329K Example 400K ? °C •400 – 273 = •127 °C Changing Temp • Increasing heat ADDED • Decreasing heat being REMOVED How is • CALORIES heat • unit of heat measured? • 1 calorie = 4.184 J • Amount of heat needed to raise the temp of 1g of water 1°C 1000 calories (1 kilocalorie) = 1 food Calorie Your body uses the calories in food for heat and energy. Some foods contain more calories than others, and therefore provide your body with a concentration of energy. Foods that are high in calories contain large amounts of chemical energy, often more than your body can properly break down and use. What's left over is often stored as fat. That's why people on diets avoid foods that are high in calories. TOPIC: Heat AIM: What is thermal expansion? Thermal • Expansion (increase Expansion in size) of a substance caused by heat • Temp increases molecules move faster & farther apart • Examples: • 1. The rising of mercury/ alcohol in a thermometer • 2. • Hot air balloons rise because heated air expands When both ball and ring are at room temperature, the ball fits through the ring. When the ball is heated, it no longer fits due to thermal expansion. Spaces between the joints allow the metals in the bridge to expand. Summary: • Recap lesson • Read article on thermometer and absolute zero • Paraphrase partners? • http://www.brainpop.com/science/energy/h eat/zoom.weml











