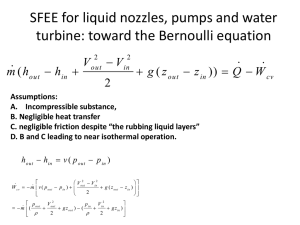
Week 5 First Law of Thermodynamics 1 First Law of Thermodynamics ⚫Conservation of energy principle: ⚫Energy can be neither created nor destroyed; ⚫it can only change forms. ⚫Conservation of energy the total amount of energy in an isolated system remains constant 2 The 1st law the application of the conservation of energy principle to heat and thermodynamic processes; e.g ⚫-the kinetic energy of a moving car is converted into heat energy at the brakes and tire surfaces. ⚫-when chemical energy is released in burning and is converted into light and heat energy. ⚫-an object can be lifted by heating a rubber band. Heat is converted into gravitational potential energy 3 NOTE: ⚫Process changes in the properties of that system. ⚫When a very small part of a process occurs in a system, and is accompanied by a correspondingly very small change in the properties of the system ⚫-A large (finite) change in the quantity X ⚫-A small (infinitesimal) change in X dX ΔX 4 ⚫Q: -denotes heat, it has units of energy (it is an energy interaction): Joules (J) or kJ (kilo-Joules). -energy in transit due to the thermal interaction (temperature difference between the system and its surroundings). ⚫W: -denotes work interaction, it has units of energy (it is also an energy interaction): Joules (J) or kilo-Joules (kJ). -energy in transit due to mechanical interaction between the system and its surroundings ⚫E: -Energy contained by the system (stored energy), property of the system. ⚫How much work can we possibly get out of heat? 5 6 Energy balance ⚫E: represents the change in energy experienced by the system. If the system experience a change from state 1 to state 2, then In many cases in thermodynamic analysis 7 8 9 10 11 12 13 14 c 15 ⚫Let say u = f(T,v) ⚫Integratin g 16 c 17 18 19 ⚫means T2 = 300 Κ= T1 ⚫means Q = 0 P2 =? T2 = 300K V2 = 0.05 m3 +0.03 m3 = 0.08 m3 20 Example: Throttling Valves Throttling valves are any kind of flowrestricting devices that cause a significant pressure drop in the fluid. The pressure drop in the fluid is often accompanied by a large drop in temperature, and for that reason throttling devices are commonly used in refrigeration and air-conditioning applications. 21 22 23 The temperature of an ideal gas does not change during a throttling (h = constant) process since h = h(T). During a throttling process, the enthalpy of a fluid remains constant. But internal and flow energies may be converted to each other. Energy balance 24 5–58C Why are throttling devices commonly used in refrigeration and air-conditioning applications? 5–59C Would you expect the temperature of air to drop as it undergoes a steady-flow throttling process? Explain. 5–60C Would you expect the temperature of a liquid to change as it is throttled? Explain. 5–61C During a throttling process, the temperature of a fluid drops from 30 to 20 °C. Can this process occur adiabatically? 5–62 Refrigerant-134a is throttled from the saturated liquid state at 700 kPa to a pressure of 160 kPa. Determine the temperature drop during this process and the final specific volume of the refrigerant. Answers: 42.3 °C, 0.0345 m3/kg 25 Example: Heat exchangers ⚫Heat exchangers are devices where two moving fluid streams exchange heat without mixing. Heat exchangers are widely used in various industries, and they come in various designs. A heat exchanger can be as simple as two concentric pipes. 26 Mass and energy balances for the adiabatic heat exchanger in the figure is: 27 The heat transfer associated with a heat exchanger may be zero or nonzero depending on how the control volume is selected. 28 Mass and Energy balances for a steady-flow process Mass balance A water heater in steady operation. 29 5–76 A heat exchanger is to heat water (cp= 4.18 kJ/kg·°C) from 25 to 60 °C at a rate of 0.2 kg/s. The heating is to be accomplished by geothermal water (cp= 4.31 kJ/kg·°C) available at 140 °C at a mass flow rate of 0.3 kg/s. Determine the rate of heat transfer in the heat exchanger and the exit temperature of geothermal water. 5–78 A thin-walled double-pipe counter-flow heat exchanger is used to cool oil (cp= 2.20 kJ/kg·°C) from 150 to 40 °C at a rate of 2 kg/s by water (cp= 4.18 kJ/kg·°C) that enters at 22 °C at a rate of 1.5 kg/s. Determine the rate of heat transfer in the heat exchanger and the exit temperature of water. 30