Thermodynamics Quiz I
(2011. 10. 8: 205 points)
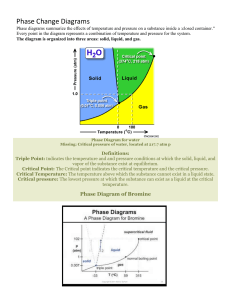
1. (a) Explain the thermodynamic characteristics of R, U, A, B and Critical
locus within one line per each in figure (10 points)
(b) Write four kinds of the criterion of equilibrium with condition. (10 points)
2. (20 points) Derive the Gibbs/Duhem equation at const. T and P from
nM=F(T,P,n1,n2.,ni.).
3. (30 points) The system acetone(1) / acetonitrile(2) / nitromethane(3) at
80℃ and 110 kPa has the overall composition, z1=0.45, z2=0.35, z3=0.20.
Assuming that Raoult's law is appropriate to this system, determine L, {xi},
and {yi} when V is 0.7364 mol. The vapor pressures of the pure species at
80℃ are:
P1sat = 195.75
P2sat = 97.84
P3sat = 50.32kPa
4. (40 points) a process tream contains light species 1 and heavy species 2.
A relatively pure liquid stream containing mostly 2 is desired, obtained by a
single-stage
liquid/vapor
separation.
Specifications
on
the
equilibrium
composition are: x1=0.002 and y1=0.950. Use data given below to determine
T(K) and P(bar) for the separator. Assume that the vapor is ideal gas; the
calculated P should validate this assumption. (Note: As a first guess, use
300K. Then, iterate once as an answer.)
For the liquid phase
ln ln
ln
(bar, K)
5.
(30
Points)
A
liquid
mixture
of
cyclohexanone(1)/phenol(2)
for
which
is in equilibrium with its vapor at 144℃. Determine the equilibrium
pressure P and vapor composition
◉
.
◉ At 144℃,
and
from the following information:
.
◉ The system forms an azeotrope at 144℃ for which
6. (10+15+10=35 Points) The excess Gibbs energy of a binary liquid mixture
at T and P is given by:
(a) Find expressions for
and
at T and P.
(b) Show that
.
(c) Plot the schematic diagram for GE/RT, ln γ1, and ln γ2 vs. x1. Show the
values of
and
. (Don't need to calculate each point.)
≡
using
at const. T & P
7. (20+10 points) For the system ethylene(1)/propylene(2) as a gas, estimate
the fugacity coefficient of
y1=0.35.
(a) Using following equations
each
component
at
t=150oC,
P=30
(b) Assuming that the mixture is an ideal solution.
ethylene
propylene
0.281
0.289
[cm mol ]
131
188.4
[K]
282.3
50.40
0.087
365.6
46.65
0.140
3
Pc [bar]
ω
-1
bar,
and
φ
⌠
⌡
(A)
ln
≡
ω
ω
ω
φ
(B)
ω
()
≡
(D)
(E)
(F)
(G)
(H)
(I)
(J)
ω
(K)