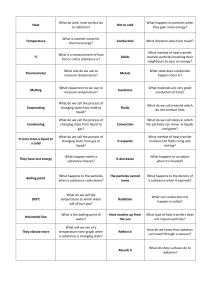
Thermal Physics • Cambridge IGCSE Combined Science pages 488-517 • Learning Outcomes, P3.1-P3.3 States of Matter Construct a summary of the three states of matter based around the given triangle. • Name the processes for the conversions between the different states. Decide if they are exothermic or endothermic processes. • By the solid, liquid and gas labels list the properties that distinguish between the states of matter. Include the forces and distances between molecules and the motion of the molecules in each state. A diagram of particles using spheres is necessary. Now for a bit of drama…… When you have finished your summary use your group members as molecules that make up a pure substance (e.g. water) and model that substance at different temperatures so that you go through the transition from solid to liquid to gas. Kinetic Molecular Model of Matter • All substances contain very small moving particles (atoms, molecules, ions) • Small particles move faster than large particles at the same temperature. • As the temperature rises, the particles have more kinetic energy and move faster. Temperature and Pressure • Temperature – The average kinetic energy of the particles is directly proportional to the temperature (absolute temp in Kelvin) of the substance. Therefore, the faster the motion of the particles the greater its temperature. • Pressure – The pressure that a gas exerts is caused by the collisions between the gas particles and their container. Measuring Temperature using Thermometers Evaporation • Evaporation – The particles in a liquid can move around each other and some particles near the surface of the liquid may have enough energy to escape into the air. When this happens, the liquid is said to EVAPORATE to form a gas. • The remaining particles then have a lower average kinetic energy, so the liquid cools down as evaporation happens. This is why sweating cools you down. The sweat absorbs energy from your skin so that it can continue to cool you down. Evaporation continued • Can you think of at least three ways to change the rate of evaporation of water? Boiling Point and Melting Point Graph Melting Point – The temperature at which a solid melts to form a liquid. Boiling Point – The temperature at which the liquid evaporates to form a gas. Thermal Expansion • Just about all materials will expand as their temperature increases. • The particles have more kinetic energy and move further apart. • This happens even in solids due to more vibrations. Applications and Consequences of Thermal Expansion Applications and Consequences of Thermal Expansion Conduction • Conduction – heat transfer by means of particle (atoms) vibrating within a material. Conduction continued • Good Thermal Conductors = metals • Why?? • They contain free moving electrons that can quickly transfer energy. • Thermal Insulators = ……… Conduction experiments https://www.savemyexams.co.uk/igcse/physics/cie/23//2-thermal-physics/2-3-transfer-of-thermal-energy/2-3-1demonstrating-conduction/ revision-notes Convection • Convection – heat transfer by mass motion of a liquid or gas. The heated particles move away from the source of the heat due to their expansion (less dense) when they are heated. • Can you draw a diagram to show convection currents from a heater, in a pot of water and at the seaside on a hot day? Radiation • Radiation – heat transfer by means of electromagnetic waves in the infrared part of the spectrum. All objects absorb and emit radiation. No medium (particles) is needed to transfer radiation – it can happen in a vacuum. Radiation Absorbers and Emitters • The Leslie’s cube shown below is filled with boiling water. • Predict which side will have the highest reading on the infra-red detector and which would have the lowest. • Complete the following sentences: • _________ ___________ surfaces are good emitters and absorbers of radiation. • _________ ___________ surfaces are poor emitters and absorbers of radiation. Recall Heat Transfer Experiments Heat Transfer – Flask and Straw Experiment • Place the flask and straw in the bowl and pour boiling water around it. Observe any changes. • Remove the flask and pour out the hot water when you have finished. • How does each part of the thermos flask reduce energy losses? • What measures could be taken to reduce energy loss in this home?