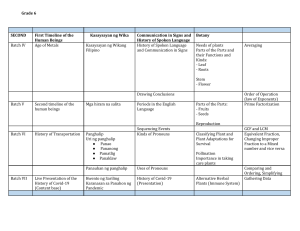
UNICURE PHARMACEUTICAL LIMITED Batch manufacturing Record Product: Page 1 of 27 Date: B.M.R. No: Batch Quantity: Reworking Added: AMLODIPINE 1.5 million tablets. Batch size: Specification: Batch no: 1.5 MILLION 5mg COMPOSITION Each film coated tablet contains: AMLODIPINE BESILATE BP Color : WHITE Theoretical yield : Mfg. date: This Document supersedes : None Reason for Change : New NAFDAC No.: Shelf Life Storage Condition Exp. Date: Material code No.: :36 Months : store in cool, dry place, away from light. Granulation Compression Date of commencement: Date of completion: Coating Area used : Previous product processed Actual yield: ________ Tablets Reworking Generated: _________kg. Total yield : ________% Packing Start date :____________ end date:________ No of cartons: _________ Final BMR Checked by QA: Final BMR Checked by : Date : Date : Checked By Reviewed By Prepared By Production Production head Quality Assurance Date : Date: Date: Date: Approved By QA %QC Head UNICURE PHARMACEUTICAL LIMITED Batch manufacturing Record Product: Page 2 of 27 Date: B.M.R. No: AMLODIPINE Batch size: Specification: Batch no: 1.5 MILLION 5mg Precaution for safety : Safety precaution to be taken against explosive in fluidized bed drier & while grinding. Protect the respiratory organs from active substance. Store the granules & tablets in well closed containers lined with double polythene bags. Notes: 1. 2. 3. 4. 5. 6. Manufacturing is to be carried out as per requirement of current GMP. Use clean and dry S.S. equipment at all stages of ,manufacturing. Carry out sifting and milling operation near dust extraction. All equipment and machinery must be adequately guarded and earthed. The operators must use proper safety equipment like gloves, nose mask,ear muffs etc. during all operation. Ensure that general cleaning &utensils are carried out as per respective S.S., FBD, screens, compression machine coating pan &tools and accessories etc. are cleaned as per respective S.C.P. & checked for cleanliness before use. 7. Ensure that RMG, V-Blender, mutimill, Vibratory sifter,S.S. Sieves,S.S., FBDS, Screens, Compression machine coating pan & tools and accessories etc. are cleaned as per respective S.C.P. 8. Before weighing operations, calibrate, center and check cleanliness of balance as per S.C.P. 9. Batch size may vary depending upon the equipment .prior permission of QA/QC should be obtained except in the case of batch in which reworking is added. 10. Destroy all rejected material & tablets by QC dept, prior to use and must be within retest date. General instruction for manufacturing Cleaning record of machinery & Equipment Sr. No. Machinery/ equipment 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. Weighing ;floor scale Analytical balance Heating tank RMG Sieves/sieve shaker Mutimill Fluidized bed dryer v- Blender Vessels, Scoops & Spatulas Compression machine 11. 12. Coating pan Tools buckets and other assessories capacity 350kg 220g 500l 250lts 500l 2000ltrs. 31/35 station 42’ - Equipment Previous No(s) Product &B. no. cleaned on By Chd. By Re-chd. By UNICURE PHARMACEUTICAL LIMITED Batch manufacturing Record Product: Page 3 of 27 Date: B.M.R. No: AMLODIPINE Batch size: Specification: Batch no: WAREHOUSE/ BUFFER ROOM –Line Cleanrance Record Previous product processed: Batch No: completed on: Sr. no Description time: Remark Yes/no 1.5 MILLION 5mg Checked by (prod.) Rechecked by (Q.A) 1. 2. 3. 4. 5. 6. 7. Label (s) of previous product Tablets/Granules of previous product Container(s) of previous product Area cleanliness(floor, walls) Grinding Machine is ready and clean properly Area is approved for operation by Q.A. o Temp. = c, Humidity = % RH Line Clearance is Satisfactory / not satisfactory. Line Cleared By (prod.) Date: Time: Checked by(QA): Date: Time: Bill of Raw Material & Weighing Record s/ No ingredients 1 2. 3. 4. 5. 6. 7. 8. 9. Amlodipine Starch Microcrystalline cellulose Hydroxpropyl cellulose Hydropromellose Starch Silicon dioxide Magnesium stearate lactose Total. Mat code T12001 T12003 T12053 T12008 T12001 T12009 T12009 T12002 Qty for batch (kg) 7.50_ 112.00 75.00 75.00 1.20 6.60 6.00 97.50 97.50 383.8 Bal. No Gross Wt. (kg) Tare Wt (kg) Net Wt (kg) Wtd by Chkd. By UNICURE PHARMACEUTICAL LIMITED Batch manufacturing Record Product: Page 4 of 27 Date: B.M.R. No: AMLODIPINE Batch size: Specification: Batch no: 1.5 MILLION 5mg COATING (FILM COATING) Bill of Raw Materials & weighing record sr. no ingredients 1 2 3 4 5 6 Hypromellose Macrogol-6000 Talcum powder Purified water Qty/ batch (kg) 2.8 0.78 2.5 85.42 Bal. no Gross Wt (kg) Requisition given by : Area cleanliness Production pharmacist: Checked by : Stores supervisor: Previous product processed: Material required on: Calculation checked by: Production: Q.A.: ATTACH DUPLICATE COPY OF WEIGHING SLIPS Tare Wt(kg) Date: Date: Date: B. No: Issued on : Date: Date: Ney Wt (kg) Weighed by Checked by UNICURE PHARMACEUTICAL LIMITED Batch manufacturing Record Product: Page 5 of 27 Date: B.M.R. No: AMLODIPINE Batch size: Specification: Batch no: NAME OF PRODUCT: Amlodipine besilate tablets (10mg) Pretreatment Record Line clearance type: Same APL, Has line clearance expired: yes no Room : FZB-300 milling machine vibrating sieve, Batch number: Different APL, Electronic balance Operator :__________ checked by:__________ QA checked by:__________ The number of sieving mesh:__________________ Completeness test before operation of sieve circle in the the machine complete incomplete Checked by :_________________________ 1.5 MILLION 5mg date:_____________ date: ________________________ Pretreatment Record Name of material Manufacture SOP 1.5 million pieces Amlodipine Treatment (no mesh), 60 mesh 1.0 mm treatment , 60mesh Nylon treatment ,60mesh Nylon screen treatment 80 mesh Nylon screen treatment 60 mesh Wire-mesh treatment, 20mesh Wire-mesh treatment ,20 mesh Wire-mesh treatment , 20 mesh Wire-mesh treatment , 20mesh 7.50_ Starch Microcrystalline cellulose Hydropropyl cellulose Hydromellose Starch Silicon dioxide Magnesium stearate lactose Weight after treatment(kg) 112.00 75.00 75.00 1.20 6.60 3.00 6.00 97.50 Operator: ____________ reviewed by:______________ QA checked by :____________ date:____________ Attach line clearance certificate of previous batch ( only for different API) Actual yield rate % UNICURE PHARMACEUTICAL LIMITED Batch manufacturing Record Product: Page 6 of 27 Date: B.M.R. No: No Batch size: Specification: Batch no: 1.5 MILLION 5mg Name of products: Amlodipine Besilate Tablets (10mg) Batch number: Material distributing record (divide the batch into 8 circle) 1st Name of materials Purpose Theory Quality/per 2nd 3rd 4th Quality in (kg) cycle 1 Amlodipine 2 3 starch Microcrystalline cellulose Hydropropyl cellulose Hydromellose Starch Silicon dioxide Magnesium stearate Lactose 4 5 6 7 8 9 10 11 12 AMLODIPINE Total weight Operator by/ date: Weigh record Rejected quantity : -------------kg Yield rate standard (%) Operator:_________ Active 7.50_ ingredient 112.0 75.00 1.86 Excipients 75.00 1.20 6.60 3.00 6.00 97.50 18.6 0.3 1.65 0.75 1.5 24.38 383.8 …………… ……………. 94.86 Lubricant 5th 6th 7th 8th 28 18.6 reviewed by: Actual yield rate (%) workshop personnel:____________ Material balance standard A (%) 99.00<A<101.00 Actual material balance (%) QA personnel:______________ Date :_______________ UNICURE PHARMACEUTICAL LIMITED Batch manufacturing Record Product: Page 7 of 27 Date: B.M.R. No: AMLODIPINE GRANULATING ROOM – line Clearance Record Same APL: Sr. Previous product:Operation Batch no : Completed on: No. Sr. No. Description 1.0 Batch size: Specification: Batch no: Different Date & timeAPL Time: Remark From Checked To by yes/No (prod) PREPARATION OF GRANULATION SOLUTION LabelPRODUCTION (s) of previousBATCH product FOR ONE Tablets/Granules of previous Add 1.2g of Hypromellose to 1.2kgproduct of hot purified water, stir evenly Container(s) of previous product to make a slurry. Add slurry to 10kg of hot purified water and stir Area cleanliness(floor, walls) evenly. Add 28.8kg of purified water at room temperature to make a Machinedivide is ready clean properly 40kgGrinding binder solution. intoand 8 portions. Area is approved for operation by Q.A. Add 6.6kg of starch, add 19kg of purified water at room Temp. = with stirring °C, Humidity %RHwithout lumps. Add temperature to make=a solution Clearance is Satisfactory not satisfactory. boiling purified water up toLine 82.5kg to make starch slurry./ Divide into Line Cleared By (prod.) Date: Time: 8 portion Checked by(QA): Date: Time: Each lot: Hypromellose 5kg Starch 13.50kg 2.0 MIXING AND GRANULATION: 2.1 Transfer into cleaned RMG bowl raw material and excipients for lot. 2.2 Mix for 4 minutes atklklation of stage 2.1, in a thin stream into mixer, while mixing at slow speed within 4 min. then mix for 3 min. on fast speed. Scrape and mix for 1 min. with agitator and chopper “ON” to get suitable consistency of granulation. Continue the mixing further on slow speed. Total mixing time----------------------Transfer the granules to FBD bowl through the discharge port. Keep the agitator at slow speed & chopper in OFF position during transferring the wet mass. 3.0 WET MASS MILLING: Check the integrity of the sieve before & after of individual material. Pass the wet mass at through oscillating granulator using MESH NO. 16 at medium speed. 1. 1.1 2. 3. 4. 5. 6. 7. 4.0 DRYING: Dry the wet mass at stage 5.0 in a FBD at 70+ 2o c. for 10-15 minutes. Rake over intermittently so that all material in contact with the screen is turned. Inlet temperature:……………………….( < 85oc) 1.5 MILLION 5mg Operation Rechecked Done by by: Chd. By (QA) UNICURE PHARMACEUTICAL LIMITED Batch manufacturing Record Product: Page 8 of 27 Date: B.M.R. No: AMLODIPINE Batch size: Specification: Batch no: 1.5 MILLION 5mg Outlet Temperature : ………………………(<60oc) Total time taken for drying:…………………….. 5.0 The drying is adequate when the LOD reaches 2.5 % or moisture content 2.0- 4.0 % at 70℃ SIZING & SIFTING: Check the completeness of sieve before use Grind the coarse material through oscillating granulator using 14 mesh screen. Redry if required and repeat stage Note & record yield of dried granules Std. yield: 99.50%, permissible yield: 99.00-100.00% Attach weighing slips Process sheet Sr Operation No. 6.0 REWORKING: Batch no: Mfg. date: Exp. Date: Quantity Date & time of operation operation From Done by To Chd by UNICURE PHARMACEUTICAL LIMITED Batch manufacturing Record Product: Page 9 of 27 Date: B.M.R. No: 7.0 7.1 7.2 7.3 7.4 7.5 8.0 AMLODIPINE Batch size: Specification: Batch no: 1.5 MILLION 5mg The total qty. of reworking added should not exceed 5% of actual batch size. The reworking to be added should be crushed through 2 mm screen using the pin mill & sift through 20#. Add the reworking to the bulk of blending. Reworking generated should be use within 3 month of its generation. LUBRICATION: Transfer granules of stage 7.0 & reworking of stage 8.0 if any to the vblender by vacuum suction & mix for 5min. Sift through 40# meshMagnesium stearate :4.8 kg Transfer the lubricant of stage 9.2 to blend of 9.1 by sandwich method & mix for 10 minutes. Collect the lubricated granules in double polythene bag & record wt. theoretical weight=………….kg + weight of reworking=…………kg Std. yield : 99.50% permissible yield : 99.00-100.00% SAMPLING: collect adequate samples at specified intervals as per SOP and send to QC lab for in process testing. Compression: Compress the lubricated granules into tablets as per given specification. Container 1. 2. 3. 4. 5. 6. 7. 8. Gross wt.(kg) Tare wt. (kg) Net wt. (kg) Signature UNICURE PHARMACEUTICAL LIMITED Batch manufacturing Record Product: Page 10 of 27 Date: B.M.R. No: AMLODIPINE Batch size: Specification: Batch no: 1.5 MILLION 5mg 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. Signature Of Production Pharmacist: _______________ Lubricated Granules output: ------------------ date: ______________ Balance ID No: ----------------- Yield Rate of Granulation and Material Balance (unit: kg) Yield rate(%)= __________total weight of granules ×100%_______ Total weight of raw materials and excipients Material balance e (%) = total dry granules weight + rejected quantity + Sampling quantity×100% Total weight of raw materials and excipients Line clearance Record ( compression) Tablet compression machine – Line Clearance Record Previous product compressed: Batch no: completed on: Sr. Description No. 1. Label (s) of previous product 2. Tablets/Granules of previous product Remark yes/No time: Checked by (prod) Rechecked by: (QA) UNICURE PHARMACEUTICAL LIMITED Batch manufacturing Record Product: Page 11 of 27 Date: B.M.R. No: AMLODIPINE Batch size: Specification: Batch no: 3. Container(s) of previous product 4. Area cleanliness(floor, walls) 5. compression Machine is adjusted properly 6. Area is approved for operation by Q.A. 7. Temp. = c, Humidity = %RH Line Clearance is Satisfactory / not satisfactory. no Line Cleared by (prod.) Date: Checked by(QA): Date: 1.5 MILLION 5mg Line clearance expired: yes Time: Time: Attach line clearance certificate Container 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. Net wt./kg) Signature/Time Container 12. 13. 14. 15. 16. 17. 18. 19. 20. 21. 22. Total : Net wt. (kg) Machine used Operator Last product processed: Cleanliness check on the machine B. No: & area Punches and dies appearance Cleanliness check for punches Balance check Shift date Time started Description compressed tablets: round, white to off white tablets Signature/Time UNICURE PHARMACEUTICAL LIMITED Batch manufacturing Record Product: Page 12 of 27 Date: B.M.R. No: AMLODIPINE Batch size: Specification: Batch no: 1.5 MILLION 5mg R STD UNITS 0.3989/tablet 7.978g±2.0 % grams 3.0 7 ̴ .0 +4.12 NMT 15 NMT 1.0 Kg/cm2 mm Min % PERIODIC CHECKS AT COMPLETE ROTATION SR NO. 1. 2. 3. 4 5. 6. 7. 8. 9. 10. 11. OPERATION L Setting of machine Checking complete rotation Weight adjustment: per Tablet 20 tablets Appearance Hardness Thickness Disintegration time Friability (wt. of Tabs. Corresponding about 6.5 gm.) Before rotation Machine speed Temperature Humidity SIGNATURE 10~22 R.P.M. 18~28OC 55-65% Attach duplicate copy of previous line clearance certificate. (only for different API.) TABLET COMPRESSION RECORD NAME OF PRODUCT_______________________ A VERAGE WEIGHT RANGE_____________________ Specification: _________ Batch No: ____________ Room No: _____________ Date: ________________ : _____________________ Operator: _______________ UNICURE PHARMACEUTICAL LIMITED Batch manufacturing Record Product: Page 13 of 27 Date: B.M.R. No: Sr. No AMLODIPINE Batch size: Specification: Batch no: 1.5 MILLION 5mg Left Time Right Mach No. Individual wt. 0.3989g/Tab. Thickness <4.12 Time Mach Individual wt. No. 0.3989g/Tab. 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 Name of Product: Metformin HCL Tablets(500mg) Batch Size: 1.5million pieces Die Specification: Speed: 12- 22 rpm Hardness standard: >3kg Thickness standard: <5.68mm Tablets Weight in theory : Batch Number: Compressing Date: Machine No: Operator: Average tablets weight range: Shift: Room No: Thickness <4.12 UNICURE PHARMACEUTICAL LIMITED Batch manufacturing Record Product: Page 14 of 27 Date: B.M.R. No: AMLODIPINE Batch size: Specification: Batch no: 1.5 MILLION 5mg Upper limit of average tablets weight Lower limit of average tablets weight Unit: g Every block in ordinate equals……………..mg every 2 blocks in abscissa equals ………………… minutes DISINTEGRATION TEST RECORD (Disintegration machine No ___________________)_ Disintegration time limit standard: _______________________Test time: ________________________ Test result: ______________ minutes WEIGHT DIFFERENCE : standard limit+ 4.5% Average Weight: Weight Differences: Test Time: TABLET COMPRESSION RECORD NAME OF PRODUCT_______________________ A VERAGE WEIGHT RANGE_____________________ Specification: _________ Batch No: ____________ Room No: _____________ Date: ________________ : _____________________ Operator: _______________ UNICURE PHARMACEUTICAL LIMITED Batch manufacturing Record Product: Page 15 of 27 Date: B.M.R. No: Sr. No AMLODIPINE Batch size: Specification: Batch no: 1.5 MILLION 5mg Left Time Right Mach No. Individual wt. 0.3989g/Tab. Thickness <4.12 Time Mach Individual wt. No. 0.3989g/Tab. 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 Name of Product: Metformin HCL Tablets(500mg) Batch Number: Batch Size: 1.5million pieces Die Specification: Compressing Date: Speed: 12- 22 rpm Machine No: Hardness standard: >3kg Operator: Thickness standard: <5.68mm Shift: Room No: Thickness <4.12 UNICURE PHARMACEUTICAL LIMITED Batch manufacturing Record Product: Page 16 of 27 Date: B.M.R. No: AMLODIPINE Tablets Weight in theory : Upper limit of average tablets weight Batch size: Specification: Batch no: 1.5 MILLION 5mg Average tablets weight range: Lower limit of average tablets weight Unit: g Every block in ordinate equals……………..mg every 2 blocks in abscissa equals ………………… minutes DISINTEGRATION TEST RECORD (Disintegration machine No ___________________)_ Disintegration time limit standard: _______________________Test time: ________________________ Test result: ______________ minutes WEIGHT DIFFERENCE : standard limit+ 4.5% Average Weight: Weight Differences: Test Time: TABLET COMPRESSION RECORD Specification: _________ Batch No: ____________ Room No: _____________ UNICURE PHARMACEUTICAL LIMITED Batch manufacturing Record Product: Page 17 of 27 Date: B.M.R. No: AMLODIPINE Date: ________________ Batch size: Specification: Batch no: : _____________________ 1.5 MILLION 5mg Operator: _______________ NAME OF PRODUCT_______________________ A VERAGE WEIGHT RANGE_____________________ Sr. No Left Time Right Mach No. Individual wt. 0.3989g/Tab. Thickness <4.12 Time Mach Individual wt. No. 0.3989g/Tab. 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 Name of Product: Metformin HCL Tablets(500mg) Batch Number: Batch Size: 1.5million pieces Die Specification: Compressing Date: Speed: 12- 22 rpm Machine No: Shift: Room No: Thickness <4.12 UNICURE PHARMACEUTICAL LIMITED Batch manufacturing Record Product: Page 18 of 27 Date: B.M.R. No: AMLODIPINE Hardness standard: >3kg Thickness standard: <5.68mm Tablets Weight in theory : Upper limit of average tablets weight Batch size: Specification: Batch no: 1.5 MILLION 5mg Operator: Average tablets weight range: Lower limit of average tablets weight Unit: g Every block in ordinate equals……………..mg every 2 blocks in abscissa equals ………………… minutes DISINTEGRATION TEST RECORD (Disintegration machine No ___________________)_ Disintegration time limit standard: _______________________Test time: ________________________ Test result: ______________ minutes WEIGHT DIFFERENCE : standard limit+ 4.5% Average Weight: Weight Differences: Weight Variation Record Machine ID No:__________ Date:_________________ Balance ID No: Shift:____________________ Wt. variation Limit: +2.5 % of Avg. Wt. Test Time: UNICURE PHARMACEUTICAL LIMITED Batch manufacturing Record Product: Page 19 of 27 Date: B.M.R. No: Wt. of 20 Tablets Wt. wt. of Tab Time in hours Weight of individual Tablets in mg. AMLODIPINE g Mg g Mg Batch size: Specification: Batch no: g Mg g Mg 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. Max. Wt. Allowed Actual Min. Wt. Allowed Actual FRIABILITY TEST: Wt. of Tabs Corresponding about 6.5 gm. Before Rotation Wt. of Tabs. After 100 Rotations % loss Sign. Date QA In process control report Balance ID No: Date of Compression Shift g Mg 1.5 MILLION 5mg g Mg g Mg g Mg g Mg UNICURE PHARMACEUTICAL LIMITED Batch manufacturing Record Product: Page 20 of 27 Date: B.M.R. No: AMLODIPINE Batch size: Specification: Batch no: Mach. Used No. of punches Theoretical weight of 20 Tablets Time Actual wt. of 20 Tablets L R 1.5 MILLION 5mg Operator Punches used Wt. variation limit Disintegration Time Hardness Thickness NMT 15 min. NLT 3~7kg./cm2 < 4.12 L L R R L ±2% OF 20 TABLETS Operator R Check & record the weight of 20 tablets every 15 minutes. Check & record the average hardness of 3 tablets & average thickness of 3 tablets every 30 mins. Check & record the D.T. of 6 tablets, weight variation & friability of tablet every 2 hours. Balance ID No: Chd. By UNICURE PHARMACEUTICAL LIMITED Batch manufacturing Record Product: Page 21 of 27 Date: B.M.R. No: Container 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. 21. 22. 23. 24. 25. 26. 27. 28. 29. 30. AMLODIPINE Gross wt. (kg) Batch size: Specification: Batch no: Tare wt. (kg) Net wt. (kg) 1.5 MILLION 5mg Sign UNICURE PHARMACEUTICAL LIMITED Batch manufacturing Record Product: Page 22 of 27 Date: B.M.R. No: AMLODIPINE Total wt in kg: Batch size: Specification: Batch no: 1.5 MILLION 5mg No of tablets: Theoretical yield :……………………………..kg + Reworking added:………………………kg. Total yield:……………………….. Yield standard :99.00% Actual yield :………………………… Material Balance Compressed tablet yield rate and material balance calculation Material balance equation (%)= total tablets weight + rejected quantity + sampling quantity ×100% Total weight of granules Yield rate = total tablets weight ×100% Total weight of granules To be filled by Quality Assurance (Uncoated Tablets) Appearance of the tablet Average weight of the tablet: …………..mg. Hardness = ……………………….kg/cm2 Disintegration time = …………..min Thickness = ………mm. Friability = ………… Date of manufacturing : Remarks: Approved / Not Approved for further processing Analyst: Date: Date of expiry : UNICURE PHARMACEUTICAL LIMITED Batch manufacturing Record Product: Page 23 of 27 Date: B.M.R. No: AMLODIPINE Tablet Coating – Line Clearance Record Previous product compressed: Batch No: Completed on: Sr. No. Description Batch size: Specification: Batch no: Shift Remark Yes/No 1.5 MILLION 5mg Time: Checked by (prod) Rechecked by ( Q.A.) 1. 2. 3. 4. 5. Label (s) of previous product Tablets/Granules of previous product Container(s) of previous product Area cleanliness(floor, walls) Coating Machine is operational and properly cleaned 6. Batch is approved for operation by Q.A. 7. Temp. = ℃, Humidity = % RH Line Clearance is Satisfactory / not satisfactory. Line Cleared by (prod.) Date: Time: Checked by(QA): Date: Time: Process Sheet (Coating) Sr. No. 11.0 11.1 11.2 12.0 12.1 Operation Preparation of coating solution: Under constant mechanical stirring disperse by sprinkling, In 95% ethanol 300kg, of Orange colored coating material: 24kg Then add 0.462kg purified water . Stir for 10 minutes to get a homogeneous slurry. Filter the solution through 200# nylon cloth and weigh Coating Load the dedusted & inspected core tablets into a clean, dry S.S. coating pan. Date &time of operation From To Operation Done by Chd. By UNICURE PHARMACEUTICAL LIMITED Batch manufacturing Record Product: Page 24 of 27 Date: B.M.R. No: 12.2 12.3 12.4 12.5 12.6 12.7 12.8 AMLODIPINE Batch size: Specification: Batch no: ADJUST: RPM of coating pan ( 4- 8 RPM) Temperature of hot air blower: 45 – 55oc. Air pressure: 0.4 – 0.5 MPa Peristaltic pump: 70 to 80 RPM Apply the film coating solution to tablets using a clean spray gun assembly. After completion of coating rotate the pan for drying the tablets in pan for 10 minutes at 40oc. At the end of coating, record weight of 100 tablets. Collect the tablet in double polyethylene bags. Intimate Q.A. Department to collect the representative sample for testing. Check & record net weight of coated tablet. Keep the bag tightly closed with proper labels. Before taking tablets for packing, inspect the tablet for spotted appearance, black specs, broken & chipped tablet. Segregate the rejection as reusable and to be destroyed, separated & note down the weights. Accordingly & store the tablets in double polythene bags. ………RPM ……………oc …………kg./cm2 …………..RPM Time From: To: Temp………..oc Wt.:…………gm Wt.:…………kg 1.5 MILLION 5mg UNICURE PHARMACEUTICAL LIMITED Batch manufacturing Record Product: Page 25 of 27 Date: B.M.R. No: AMLODIPINE Batch size: Specification: Batch no: 1.5 MILLION 5mg Coated tablet check Appearance: Weight. of 100 tablets: ……………………..gm. Standard /Tab + 5.0 % w/w NMT 15 minutes. <4.12 mm Average weight: Disintegration time : Thickness: Check by & Date : Observed Tablet Output Form (Coated) Balance ID No: Container No. Gross wt.(Kg.) Tare wt. (Kg.) Net wt.(Kg.) Sign. 1. 2. 3. 4. 5. 6. 7. 8. Total wt in kg:…………. Yield Standard : 99.00% Check by: No of tablets:………………… Actual yield :……………….. QA…………………………. Total Theoretical yield :……………. Date:………………………………….. Inspection ( Coated Tablets) Inspect the tablet for black spots, foreign particles, chipped or broken tablets. Etc. A Record the weight of rejected tablets i.e. black spots, foreign particles, chipped or broken tablets and put in water for destruction. Weight of tablets=………………kg B Chipped and broken tablets, generated during coating can be used as utilizable residue in next batch. Transfer the inspected tablets to double polythene bags inside the suitable airtight contains. Record the weight of inspected tablets. Weight of tablets= ………………..kg Tablet inspection and rejection record (coated) Area clearance checked by: Date: Shift: Previous product processed: B. No: Production pharmacist – Q.A. chemistTimeFrom: Date – Date To UNICURE PHARMACEUTICAL LIMITED Batch manufacturing Record Product: Page 26 of 27 Date: B.M.R. No: AMLODIPINE Batch size: Specification: Batch no: Total weight of tablets received for inspection= …………………. Kg. Inspection carried out by : Wt. of tablets after inspection :……………………… Balance ID No: Container No. Gross wt. Tare wt. Net wt. Kg. Kg. Kg. 1. 2. 3. 4. 5. 6. 7. 8. 1.5 MILLION 5mg Sign Total wt: ……………….. A) Reusable :…………………………kg No of Tablets: ……………………. B) Rejects to be destroyed : ……………….kg. No of tablets: ……………………. Pharmacist : Date : Rejection & Destruction Record Qty. destroyed Rejection generated during tablet compression/inspection Destruction witnessed by: Production pharmacistQ.A. Chemist- Date –Date Batch Reconciliation Data Manufacturing Date of manufacturing: Standard Batch Size =…………………kg. Reworking added =…………… Theoretical Yield =……………….. Actual Yield =…………………..kg = …………………….% Variance = ……………..kg Pharmacist signature: Date: Method used Compression Date of compression: Compression wt. =…………………………..mg Actual Yield (A)=……………………………kg. =…………………. Tablets Reworking generated (B)= ……………… Theoretical Yield (T) = ……………………. Total Yield (A+B) =……………………kg. % yield = (A+B) / T X 100 = ………….% Variance = …………………kg. Pharmacist signature : Date: UNICURE PHARMACEUTICAL LIMITED Batch manufacturing Record Product: Page 27 of 27 Date: B.M.R. No: AMLODIPINE Batch size: Specification: Batch no: 1.5 MILLION 5mg Yield statement Sr. No. STATE OF OPERATION INPUT (KG) A ACTUAL WT. KG B PERCENTAGE (%) B/A X 100 STD. YIELD (%) PERMISSIBLE YIELD (%) LOWER 1. 2. 3. 4. Dried Granules Lubricated granules Compressed tablets Coated Tablet Checked by : Date: History of Change Sr. No. Revision No./ Date 1. This Document supersedes N.A. Reason for change New HIGHER