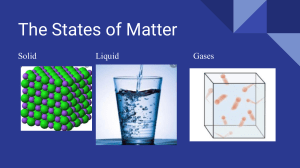
CHAPTER 5 THERMAL PROPERTIES AND TEMPERATURE Overview of IGCSE Syllabus Core: 1. Describe, qualitatively, the thermal expansion of solids, liquids, and gases at constant pressure 2. Describe some of the everyday applications and consequences of thermal expansion 3. Know that a rise in the temperature of an object increases its internal energy Supplement: 1. Explain, in terms of the motion and arrangement of particles, the relative order of magnitudes of the expansion of solids, liquids and gases as their temperatures rise 2. Describe an increase in temperature of an object in terms of an increase in the average kinetic energies of all of the particles in the object 3. Define specific heat capacity as the energy required per unit mass per unit temperature increase; recall and use the equation ∆𝐸 𝑐= 𝑚∆𝜃 4. Describe experiments to measure the specific heat capacity of a solid and a liquid 5. Recall and use the equation pV = constant for a fixed mass of gas at constant temperature, including a graphical representation of this relationship Khizar Yousaf Khizaryousaf02@gmail.com CHAPTER 5 THERMAL PROPERTIES AND TEMPERATURE Thermal expansion: • • When (most) materials are heated, they expand This expansion happens because the molecules start to move around (or vibrate) faster, which causes them to knock into each other and push each other apart When a solid is heated, the molecules vibrate more, pushing each other apart Note: When this happens, it is the space taken up by the molecules that increases. The molecules themselves remain the same size. Thermal Expansion in Solids, Liquids & Gases: • When solids, liquids and gases are heated: State Solid Liquid Gases Explain Expand slightly (Due to the strong bond holding the molecules together) Expand more than solids (Due to the weaker bonds between the molecules) Expand significantly (Due to there being no bonds holding the molecules together) Thermal Expansion in solids: “When a solid is heated, its atoms vibrate faster about their fixed points.” The relative increase in the size of solids when heated is therefore small. Metal railway tracks have small gaps so that when the sun heats them, the tracks expand into these gaps and don't buckle. Khizar Yousaf/Zeeshan Raza Page 1|6 CHAPTER 5 THERMAL PROPERTIES AND TEMPERATURE Uses of solid expansion: • In the kitchen, a tight metal lid can be removed from a glass jar by immersing the lid in hot water so that it expands and loosens. Allowing for expansion and Contraction: • Gaps used to be left between the lengths of railway lines to allow for expansion in summer. This gives a smoother journey and allows some expansion near the ends of each length of rail. • Gaps are left at the ends of bridges to allow for expansion. One end of the bridge is often supported on rollers so that movement can take place. Steel rods can be used to reinforce concrete because both materials expand equally. If the expansions were different, the steel might crack the concrete on a hot day. • • When overhead cables are suspended from poles or pylons, they are left slack, partly to allow for the contraction that would happen on a very cold day. Khizar Yousaf/Zeeshan Raza Page 2|6 CHAPTER 5 THERMAL PROPERTIES AND TEMPERATURE Bimetallic strip: • If equal lengths of two different metals, such as copper and iron, are riveted together so that they cannot move separately, they form a bimetallic strip. • When heated, copper expands more than iron and to allow this the strip bends with copper on the outside A bimetallic strip is also used in this way to work the flashing direction indicator lamps in a car, being warmed by an electric heating coil wound round it. Expansion in Liquids: Liquids expand when their atoms vibrate faster about their fixed points, but because the bonds between separate molecules are usually less tight, they expand more than solids. Unusual expansion of water: As water is cooled to 4°C it contracts, as we would expect. However, between 4°C and 0°C it expands, surprisingly. Water has a maximum density at 4°C. At 0°C, when it freezes, a considerable volume expansion occurs and every 100 cm3 of water becomes 109 cm3 of ice. This accounts for the bursting of unlagged water pipes in very cold weather. The unusual (anomalous) expansion of water between 4°C and 0°C explains why fish survive in a frozen pond. The water at the top of the pond cools first, contracts and being denser sinks to the bottom. Warmer, less dense water rises to the surface to be cooled. When all the water is at 4°C the circulation stops. If the temperature of the surface water falls below 4°C, it becomes less dense and remains at the top, eventually forming a layer of ice at 0°C. Expansion of liquid-in glass thermometer: In the liquid-in-glass thermometer the liquid in a glass bulb expands up a capillary tube when the bulb is heated. Khizar Yousaf/Zeeshan Raza Page 3|6 CHAPTER 5 THERMAL PROPERTIES AND TEMPERATURE Expansion in gases: Particles in gases move more quickly when heated. As the pressure increases, the gas particles contact the vessel surfaces more often, resulting in the thermal expansion in gases expanding and escaping the container more easily. Thermal expansion of gases is the increase in volume of a given amount of gas, as a result of an increase in temperature. The molecules or atoms comprising the gas gain kinetic energy and hence move faster. This leads to an increased amount of collisions with the walls of the container and a higher pressure. The walls of the container, if flexible, will expand as the molecules are also moving further - distance wise, and hence taking up more space - due to the rise in kinetic energy. • The most common way in which you could observe thermal expansion of gases would be if you left a balloon outside, on a hot day. As the Sun rises and the day gets hotter, you would notice the balloon expanding, as the pressure inside the balloon increases, and hence the volume. Absolute zero Scale: If the temperature of a gas is reduced, the particles have less energy and move more slowly. Eventually, at a particular temperature, the particles stop moving completely. This temperature is the lowest possible temperature and is known as absolute zero. Absolute zero is -273oC. This is also known as zero kelvin, or 0 K. To convert from oC to K, just add 273. Temperature: Temperature is the degree of hotness or coldness of a body. To measure the temperature, we are using thermometers. Khizar Yousaf/Zeeshan Raza Page 4|6 CHAPTER 5 THERMAL PROPERTIES AND TEMPERATURE Features of liquid-in-glass thermometers: Sensitivity. - The narrower the tube, the more the liquid inside moves, making the thermometer more sensitive to changes in temperature. Alcohol expands more than mercury, so a mercury thermometer must have a narrower tube than an alcohol one. Sensitivity can be increased by: a) Narrowing the bore(capillary tube) b) Using a bigger glass bulb c) Using a liquid with higher expensiveness Range – mercury has a freezing point of -39oC and a boiling point of 356oC. Alcohol freezes at -115oC, but boils at 78oC. Range can be increased by: a) Increasing the length of bore (capillary tube). b) Using a wider bore. c) Using a liquid with lower expansiveness. Note that: Factors that increases range reduces sensitivity i.e. the longer the range, the lower the sensitivity. Responsiveness – thermometers with large bulbs or thicker glass around the bulb respond less quickly to changes in temperature. Responsiveness depends upon the following factors: It can be increased by: a) Using a thin glass bulb b) Using a liquid that conducts heat better. Linearity – the expansion of mercury is not quite linear compared with alcohol – they differ slightly, but these differences are not significant between 0oC and 100oC. Specific heat capacity: Amount of heat required to raise the temperature of 1 kg of a substance by 1°C or 1 K. Mathematically: 𝐶 𝑐=𝑚 Khizar Yousaf/Zeeshan Raza Page 5|6 CHAPTER 5 THERMAL PROPERTIES AND TEMPERATURE 𝑄 𝑐 = 𝑚∆𝑇 𝐽 S.I unit: S.I unit of specific capacity is 𝑘𝑔 𝐾 𝑜𝑟 Jkg −1 K −1 The specific heat capacity of water is 4200 Jkg −1 K −1 Measuring specific heat capacity: Water A typical experiment is shown on the right. Here, the beaker contains 0.5 kg of water: When the 100-watt electric heater is switched on for 230 seconds, the temperature of the water rises by 10 °C. From these figures, a value for the specific heat capacity of water can be calculated: energy transferred to water = mc∆T = 0.5 x c × 10 energy supplied by heater = power x time = 100 × 230 = 23 000 J SO: 0.5 x c × 10 = 23 000 Rearranged and simplified, this gives c = 4600 so the specific heat capacity of water is 4600 J/(kg °C). This method makes no allowance for any thermal energy lost to the beaker or the surroundings, so the value of c is only approximate. Aluminium (or other metal) The method is as above, except that a block of aluminium is used instead of water. The block has holes drilled in it for the heater and thermometer. As before, c is calculated from this equation: power X time = mc∆T (assuming no thermal energy losses) Khizar Yousaf/Zeeshan Raza Page 6|6