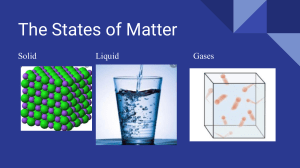
Kinetic Particle Theory Introduction The kinetic particle theory explains the properties of the different states of matter. It describes how particles move and interact in solids, liquids, and gases. Solids In solids, particles are closely packed together in a fixed position. They vibrate but do not move from their positions, which gives solids a definite shape and volume. Liquids In liquids, particles are still close together but can move around each other. This allows liquids to flow and take the shape of their container while maintaining a constant volume. Gases In gases, particles are far apart and move freely. This allows gases to fill the entire volume of their container and be easily compressed. Diagram