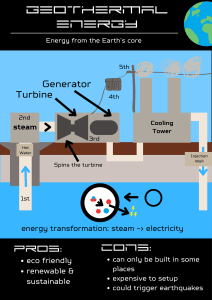
THE FIRST LAW OF THERMODYNAMICS 2.1 The First Law of Thermodynamics The first law of thermodynamics, also known as the conservation of energy that energy can be neither created nor destroyed during a process; it can only change forms, ΔEsystem + ΔEsurroundings = 0 (2.1) . Potential Energy, Kinetic Energy, and Internal Energy represent energies associated with a system. –Q –W ΔEsystem = ΔU + ΔEk + ΔEP Heat (Q) and Work (W) values, are not properties; they account for the energy changes that occur in the surroundings. + Q +W System ΔEsurrounding = ± Q ± W Surrounding Heat transfer to a system is positive (+Q) such as boiler. Heat transfer from the system is negative; (– Q) such as steam heating. Work done from a system is positive (+W) such as gas turbine. Work on a system are negative (–W) such as pump and compressor. ± Q ± W = ΔU + ΔEk + ΔEP (2.2) Internal energy (ΔU) is macroscopic properties to molecular motions and interactions. It cannot be directly measured and is stored are immaterial. Internal energy of a homogeneous system can be expressed as a function of its temperature, pressure, and composition. Absolute values are unknown in the context of classical thermodynamics that is the province of statistical thermodynamics Equilibrium state is the absence of change. Because any tendency toward change is caused by a different type of driving force as • • • Mechanical forces such as work; Temperature differences; Change phase of substances. 2.2 Energy Balance for Closed Systems Closed System: the boundary of a system does not permit the transfer of matter between the system and its surroundings, and kinetic and potential energies and mass flow are zero ± dQ ± dW = dUt Ut = nU 2.3 ± Q ± W = nΔU 2.3 Ut is the internal energy (extensive property) (kJ). U is specific internal energy (intensive property) (kJ/kg) of (kJ/kmol). In closed system undergoing a cycle, the initial and final states are ΔEsystem = 0. Exampl e 2.1 : A river flows from Lake in elevation by about 100 m at a water flow of 3,100,000 kg/s. Most of this drop occurs over fall. The hydroelectric power plant draws water from the waterfall with power capacity 2,300,000 kW. (a) What is the potential energy of the water flowing out of lake? (b) If the temperature of the water is unchanged, how much heat flows to or from it? Solution Take 1 kg of water as the system. (a) Potential energy is related to height by Eq. (1.8), EP = mzg = 1 kg × 100 m × 9.81 m/s2 = 981 kg m2 s−2 = 981 J (b) If the water leaves and enters the lake at the same temperature, then its internal energy is unchanged. Neglecting also any change in kinetic energy, The first law, Q + W = ΔEp W= J s ( وحدات الKJ/Kg )قسمة الشغل عىل معدل جريان من اجل مساوات جميع الحدود بنفس الوحدات Q = ΔEp − W = −981 + 742 = −239 J heat lost from the system. Example 2.2: A wind turbine converts about 44% of the kinetic energy of the wind approaching it into usable electrical energy. The total air flow is about 15000 kg/s with speed of 9 m/s. (a) How much electrical energy is produced when one kg of air passes through the turbine? (b) What is the power output of the turbine? (J/s = W) (c) If its temperature remains unchanged, what is its change in speed upon passing through the wind turbine? Solution on the mass basis of air (m = 1 kg) (a) the electrical energy produced per kilogram of air is 0.44 × 40.5 = 17.8 J/kg. (b) The power output is: 17.8 J/kg × 15,000 kg/s = 267,000 J/s = 267 kW (c) ΔU = 0 : If the temperature and pressure of the air are unchanged. ΔEp = 0 : if Changes in gravitational potential energy is neglected. Q = 0 : no heat transfer, the first law becomes Exampl e 2.3: When a system is taken along path acb, 100 J of heat flows into the system and the work on the system is 40 J. (a) How much heat flows into the system along path aeb if the work done on the system is 20 J? (b) The system returns along path bda. If the work done by the system is 30 J, does the system absorb or liberate heat? How much? Solution Assume that the ΔEk and ΔEp are negated تهمل. In path acb, ± Q ± W = ΔU + ΔEk + ΔEP (2.2) ΔUab = + Qacb – Wacb = 100 – 40 = 60 J (a) In path aeb, ΔUab = 60 = + Qaeb – 20 Qaeb = 80 J (b) In path bda, ΔUab = – ΔUab = + Qbda + Wbda –60 = Qbda + 30 >> heat Qbda = – 90 J Liberated Example 2.4: A gas is confined in a cylinder by a piston. The initial pressure of the gas is 7 bar, and the volume is 0.1 m3. The piston is held in place by latches. (a) The whole apparatus is placed in a vacuum. What is the energy change of the apparatus if the restraining latches are removed so that the gas expands to double its initial volume? (b) The process described in (a) is repeated, but in air at 101.3 kPa, rather than in a vacuum. What is the energy change of the apparatus? Assume the rate of heat exchange between the apparatus and the surrounding air is slow compared with the rate at which the process occurs. Solution (a) Q and W are zero, and the total energy of the system does not change. we can say nothing about the distribution of energy among the parts of the close system. (b) the system on the surroundings is a positive quantity (+𝑊); 𝑊 = 𝑃𝛥𝑉 = 101.3 (0.2 – 0.1) = 10.13 𝑘𝑁 𝑚 Q is assumed to be zero, giving: ΔEsystem = Q + W = 0 + 10.13 = 10.13 kJ 2.3THE REVERSIBLE PROCESS Reversible Process can be reversed without leaving any trace on the surroundings. That is, both the system and the surroundings are returned to their initial states at the end of the reverse process. In the cycle, the net heat and net work exchange are zero. Irreversible processes, the surroundings usually do some work on the system and therefore does not return to their original state such as friction, mixing of two fluids, electric resistance, inelastic deformation of solids, chemical reactions, heat transfer through a finite temperature difference, non-quasi-equilibrium compression. Notes on Reversible Processes • Can be reversed at any point by an infinitesimal change in external conditions • Is never more than minutely removed from equilibrium • Traverses a succession of equilibrium states • Is frictionless • Is driven by forces whose imbalance is infinitesimal in magnitude • Proceeds infinitely slowly • When reversed, retraces its path, restoring the initial state of system and surroundings The work of a gas compression or expansion caused by the displacement of a piston in a cylinder: 𝑑𝑊 = −𝑃 𝑑𝑉𝑡 𝑊𝐼𝑟𝑟𝑒𝑣𝑒𝑟𝑠𝑖𝑏𝑙𝑒 = 𝜂 𝑊𝑟𝑒𝑣 (2.5) (2.6) Example 2.5: A piston/cylinder is placed in a constant-temperature bath. The piston slides in the cylinder with negligible friction, and an external force holds it in place against an initial gas pressure of 14 bar and an initial gas volume of 0.03 m3. The gas expands isothermally as its volume doubles. If the volume of the gas is related to its pressure so that PVt = k, a) What is the final pressure? b) What is the work done by the gas in moving the external force? c) what is the actual work were the efficiency of 80%, Solution a) If PVt = k, a constant, then P = k/ Vt. b) work done c) Were the efficiency of 80%, WIrreversible = η Wrev = 0.8 × –29112 = −23290 J 2.4 Enthalpy A homogeneous fluid contained in a closed system, the energy balance of Eq. is written: 𝑑𝑈 = 𝑑𝑄 + 𝑑𝑊 The work for a mechanically reversible, closed-system process is given by Eq as written: 𝑑𝑊 = −𝑃𝑑𝑉. Substitution into the equation yields: 𝑑𝑈 = 𝑑𝑄 − 𝑃𝑑𝑉 (2.7) For a constant-volume change of state, the only possible mechanical work is that associated with stirring or mixing, which is excluded because it is inherently irreversible. Thus, 𝑑𝑈 = 𝑑𝑄 (𝑐𝑜𝑛𝑠𝑡 𝑉 ) (2.8) For a constant-pressure change of state: 𝑑𝑈 + 𝑃𝑑𝑉 = 𝑑(𝑈 + 𝑃𝑉) = 𝑑𝑄 (2.9) The group U + PV naturally arises here as a new thermodynamic property that definition of enthalpy is: 𝐻 𝑈 + 𝑃𝑉 (2.10) 𝑑𝐻 = 𝑑𝑈 + 𝑑(𝑃𝑉 ) (2.12) ∆𝐻 = ∆𝑈 + ∆(𝑃𝑉 ) (2.13) The preceding energy balance becomes: 𝑑𝐻 = 𝑑𝑄 (𝑐𝑜𝑛𝑠𝑡 𝑃) (2.14) Example 2.6: Calculate ΔU and ΔH for 1 kg of water when it is vaporized at the constant temperature of 100°C and the constant pressure of 101.33 kPa. The specific volumes of liquid and vapor water at these conditions are 0.00104 and 1.673 m3/kg, respectively. For this change, heat in the amount of 2256.9 kJ is added to the water. Solution We take the 1 kg of water as the system because it alone is of interest, 𝛥𝐻 = 𝑄 = 2256.9 𝑘𝐽 By Eq 𝛥𝑈 = 𝛥𝐻 − 𝛥(𝑃𝑉 ) = 𝛥𝐻 − 𝑃 𝛥𝑉 Then 𝛥𝑈 = 2256.9 − 101.33 𝑘𝑃𝑎 × (1.673 − 0.001) 𝑚3 𝛥𝑈 = 2256.9 − 169.4 = 2087.5 𝑘𝐽 2.5 HEAT CAPACITY 1. Specific Heat at Constant Volume Cv is the energy required to raise the temperature of the unit mass of a substance by one degree as the volume is maintained constant. 𝑑𝑈 𝐶= 𝑑𝑇 A ratio Û/ T approaches a limiting value, when ΔT→0 𝒅𝑼̂ = 𝑪𝒗(𝑻) 𝒅𝑻 2. Specific Heat at Constant Pressure Cp is always greater than Cv because at constant pressure the system is allowed to expand and the energy for this expansion work must also be supplied to the system. A ratio Ĥ/ T approaches a limiting value, when ΔT → 0 𝒅𝑯̂ = 𝑪𝑝(𝑻) 𝒅𝑻 • A unit of specific heat capacity is kJ/kg·°C or kJ/kg·K. and sometimes given on a molar basis. (kJ/kmol·°C or kJ/kmol·K). • ΔT(°C) = ΔT(K) • Cv and Cp are functions of temperature (Cp = a + bT + cT2 + dT3). Relationships between CP and Cv; 𝑑𝐻 = 𝑑𝑈 + 𝑑(𝑃𝑉) 𝑑𝐻 = 𝑑𝑈 + 𝑅 𝑑𝑇 𝑓𝑜𝑟 𝑖𝑑𝑒𝑎𝑙 𝑔𝑎𝑠 Then from above Equations. 𝐶𝑝 𝑑𝑇 = 𝐶𝑣 𝑑𝑇 + 𝑅 𝑑𝑇 𝐶𝑝 = 𝐶𝑣 + 𝑅 𝑓𝑜𝑟 𝐼𝑑𝑒𝑎𝑙 𝑔𝑎𝑠 Divide by CP 𝐶𝑃 𝑅 =1+ 𝐶𝑣 𝐶𝑣 The specific heat ratio is γ = Cp/Cv, since 𝛾 =1+ 𝑅 𝐶𝑣 ⇒ 𝑅 =𝛾−1 𝐶𝑣 𝛾 = 1.667 for monatomic gases at room temperature such as helium, neon, argon. 𝛾 = 1.4 for diatomic gases at room temperature such as air. 𝛾 = 1.3 for simple polyatomic gases such as CO2, SO2, NH3, and CH4. liquids, A substance whose specific volume (or density) is constant is called an incompressible substance. The specific volumes of solids and liquids essentially remain constant during a process. Cp = Cv = C 𝒅𝑼̂ = 𝑪 𝒅𝑻 𝒅𝑯̂ = 𝒅𝑼̂ + 𝑽𝒅𝑷 + 𝑷𝒅𝑽 𝑷𝒅𝑽 = 𝟎 𝒅𝑯̂ = 𝒅𝑼̂ + 𝑽𝒅𝑷 Two special cases are commonly encountered: 1. Constant-pressure processes, as in heaters (𝑑𝑃 = 0): 𝒅𝑯̂ = 𝑪 𝒅𝑻 2. Constant-temperature processes, as in pumps (𝑑𝑇 = 0): 𝒅𝑯̂ = 𝑽 𝒅𝑷 In solids, the term 𝑉𝑑𝑃 is insignificant and thus 𝒅𝑯̂ = 𝒅𝑼̂ = 𝑪 𝒅𝑻 Example 2.8: Calculate the internal energy and enthalpy changes resulting if air changes from an initial state of 5 °C and 10 bar, where its molar volume is 2.312 × 10−3 m3/mol, to a final state of 60°C and 1 bar. Assume that air remains a gas for which PV/T is constant and that C V = 20.785 and CP = 29.100 J/(mol·K). Solution: 1 mol of air is (a) (b) ① cooled at constant volume to the final pressure, heated final at constant pressure to the T temperature. (K) ② a b ③ T1 = 5 + 273.15 = 278.15 K T2 = 60 + 273.15 = 333.15 K The intermediate temperature between the two steps is, T2 = (278.15) (1/10) = 27.82 K the temperature changes for the two steps are: ΔTa = 27.82 − 278.15 = −250.33 K ΔTb = 333.15 − 27.82 = 305.33 K In step (a), (ΔV = 0) ΔUa = CV ΔTa = (20.785)(−250.33) = −5203.1 J ΔHa = ΔUa + V ΔPa ΔHa = −5203.1 J + 2.312 × 10−3 m3 (−9 × 105) Pa = −7283.9 J In step (b), (ΔP = 0) ΔHb = CP ΔTb = (29.100) (305.33) = 8885.1 J ΔUb = ΔHb − P ΔVb ΔUb = 8885.1 − (1 × 105) (0.02769 − 0.00231) = 6347.1 J Combined two steps is, ΔU = −5203.1 + 6347.1 = 1144.0 J ΔH = −7283.9 + 8885.1 = 1601.2 J Example 2.7: Air at 1 bar and 298.15 K, where its molar volume is 0.02479 m3/mol, is compressed to 3 bar and 298.15 K by two different closed-system mechanically reversible processes: (a) Cooling at constant pressure followed by heating at constant volume. (b) Heating at constant volume followed by cooling at constant pressure. Calculate the overall Q, W, ΔU and ΔH of the air. CV = 20.785 and CP = 29.100 J/mol·K Assume that air remains a gas for which 𝑃𝑉/𝑇 is a constant. Solution: The final volume is: 𝑃1 𝑉1 𝑃2 𝑉2 = = 𝐶𝑜𝑛𝑠𝑡 𝑇1 𝑇2 The two paths are shown on the PV diagram and the TP diagram. Final Path b P Path a Initial V (a) The temperature of the air at the end of this cooling step is: 𝑃1 𝑉1 𝑃2 𝑉2 = = 𝐶𝑜𝑛𝑠𝑡 𝑇1 𝑇2 The first step (ΔP = 0) 𝑊 = −𝑃 𝛥𝑉 = −1 × 105 𝑃𝑎 × (0.008263 − 0.02479) 𝑚3 = 1653 𝐽 𝛥𝐻 = 𝐶𝑝 𝛥𝑇 = (29.100) (99.38 − 298.15) = −5784 𝐽 𝛥𝑈 = 𝛥𝐻 − 𝛥(𝑃𝑉) = 𝛥𝐻 − 𝑃 𝛥𝑉 = −5784 + 1653 = −4131 𝐽 𝑄 = 𝛥𝑈 − 𝑊 = −4131 − 1653 = −5784 𝐽 The second step (𝜟𝑽 = 𝟎) 𝑊=0 𝛥𝑈 = 𝐶𝑣 𝛥𝑇 = 20.785 × (298.15 − 99.38) = 4131 𝐽 𝛥𝐻 = 𝛥𝑈 + 𝛥(𝑃𝑉 ) = Δ𝑈 + 𝑉 𝛥𝑃 𝛥𝐻 = 4131 + 0.008263 𝑚3 (2 × 105) 𝑃𝑎 = 5784 𝐽 𝑄 = 𝛥𝑈 − 𝑊 = 4131 − 0 = 4131 𝐽 For the overall process: 𝑊 = 1653 + 0 = 1653 𝐽 𝛥𝑈 = −4131 + 4131 = 0 ; ; Δ𝐻 = −5784 + 5784 = 0 𝑄 = −5784 + 4131 = −1653 𝐽 (b) In the first step ΔV = 0: 𝑃1 𝑉1 𝑏𝑎𝑟 𝑃2 𝑉2 𝑇1 3 bar = 𝑇2 = 𝑇2 = 𝐶𝑜𝑛𝑠𝑡 𝑉1 = 𝑉2 𝑇2 = 894.45 𝑊=0 𝛥𝑈 = 𝐶𝑣 𝛥𝑇 = (20.785)(894.45 − 298.15) = 12,394 𝐽 𝛥𝐻 = 𝛥𝑈 + 𝑉 𝛥𝑃 = 12,394 + (0.02479)(2 × 105) = 17,352 𝐽 𝑄 = 𝛥𝑈 − 𝑊 = 12394 − 0 = 12394 𝐽 In the second step, (ΔP = 0) 𝑊 = −𝑃 𝛥𝑉 = −(3 × 105)(0.008263 − 0.02479) = 4958 𝐽 𝛥𝐻 = 𝐶𝑝 𝛥𝑇 = (29.10)(298.15 − 894.45) = −17,352 𝐽 𝛥𝑈 = 𝛥𝐻 − 𝛥(𝑃𝑉) = 𝛥𝐻 − 𝑃 𝛥𝑉 = −17,352 + 4958 = −12,394 𝐽 𝑄 = 𝛥𝑈 − 𝑊 = −12394 − 4958 = −17,352 𝐽 For the overall process: 𝛥𝑈 = 12,394 − 12,394 = 0 𝑄 = 12,394 − 17,352 = −4958 𝐽 ; 𝛥𝐻 = 17,352 − 17,352 = 0 ; 𝑊 = 0 + 4958 = 4958 𝐽 2.9 Measures of Flow in Open systems Open system (control volume) is characterized by flowing streams; ṁ = ℳṅ ( 𝑘𝑔 ) 𝑠 𝑎𝑛𝑑 𝑞 = 𝑢𝐴 A specific volume ( m3 /kg ) is the density, 𝑢𝐴 (2.26) 𝑉 ṁ=𝑞𝜌=𝑢𝐴𝜌 (2.14) ṁ = 𝑞𝜌= ṁ: Mass flow rate (kg/s) ṅ: Molar flow rate (mol/s) q : Volumetric flow rate (m3/s) u: Velocity of the fluid flow (m/s) A: cross-sectional area for flow (m2). ℳ: molecular weight (kg/kmol) Continuity Equation A control volume: is the region of space identified for analysis of open systems. Continuity equation is a rate of change of mass within the control volume, dmcv/dt, equals the net rate of mass flow into the control volume, m1 dm cv /dt m2 ṁ𝒇𝒔 = ṁ𝟐 − ṁ𝟏 When ṁ is given by Eq. (2.14), dmcv/𝑑𝑡 + 𝑢2 𝐴2 𝜌32 − 𝑢1 𝐴1 𝜌1 = 0 (2.15) In a steady-state process, the accumulation term of Eq. is zero, reducing to: Δ(ρuA)𝑓𝑠 = 0 Example 2.9: In a major human artery الشريان االبهريwith an internal diameter of 5 mm, the flow of blood is 5 cm3/·s. The artery bifurcates into two identical are each 3 mm in diameter. What are the average velocity and the mass flow rate before and after of the bifurcation? The density of blood is 1.06 g/cm3. Solution before of the bifurcation, where the diameter is 0.5 cm, ṁ 𝑢𝑝 = 𝑞 𝜌 = 5 cm3/s × 1.06 g/cm3 = 5.30 g/s after of the bifurcation, the volumetric flow rate in each branch is 2.5 cm3/s, where the diameter is 0.3 cm. ṁ 𝑑𝑜𝑤𝑛= 2.5 cm3/s3 × 1.06 g/cm3 = 2.65 g/s Energy Balances for Unsteady-State Flow Processes By first Law, rate of accumulation term within the control volume is d(mU)cv/dt includes this quantity in addition to the 𝑄 and 𝑊 ate: The work rate may include work of two forms. 1. Work is associated with moving the flowing streams through entrances and exits. The work done on the system when all entrance and exit sections are −𝛥[(𝑃𝑉) 𝑚 ]𝑓𝑠. 2. Shaft work (Ws) may be associated with expansion or contraction of the control volume. Combination of terms in accord with the definition of enthalpy, H = U + PV, leads to: If kinetic- and potential-energy changes are negligible, then Eq. (2.27) simplifies to: In Steady State Process, the accumulation term of Eq. (2.27), d(mU)cv/dt, is zero, Division by 𝑚 gives: Example 2.11: an insulated tank contains 190 kg of water at 60°C. If water is withdrawn from the tank at a steady rate of ṁ = 0.2 kg/s how long will it take for the temperature to drop to 35 °C? Assume that cold water enters the tank at 10 °C and that heat losses from the tank are negligible. an assumption for liquid water is Cv = Cp = C, independent of T and P. Solution Assumptions • A perfect mixing of the contents of the tank. • A transient process (Unsteady state) 𝑑𝑈 ≠0 𝑑𝑡 • 𝑄 = 0 and 𝑊 = 0 • The input of mass flow rate equal to the output mass flow rate, • Kinetic and potential energies can be neglected. H1 is the specific enthalpy of the water entering the tank. 𝑑𝑈 𝑚 = −𝑚 (𝐻 − 𝐻1) 𝑑𝑡 𝑑𝑇 𝑚 𝐶𝑣 = −𝑚 𝐶𝑝 (𝑇 − 𝑇1) 𝑑𝑡 𝐶𝑣 = 𝐶𝑝 = 𝐶 , 𝑑𝑇 𝑚𝐶 = −𝑚 𝐶 (𝑇 − 𝑇1 ) 𝑑𝑡 𝒕 = 𝟔𝟓𝟖. 𝟓 𝒔 = 𝟏𝟏 𝒎𝒊𝒏 Example 2.13: Air at 1 bar and 25°C enters a compressor at low velocity, discharges at 3 bar, and enters a nozzle in which it expands to a final velocity of 600 m/s at the initial conditions of P and T. If the work of compression is 240 kJ/Kg, how much heat must be removed during compression? Solution: • Because the air returns to its initial conditions of T and P, an enthalpy is no change. • The potential-energy change of the air is presumed negligible. • Neglecting also the initial kinetic energy of the air, Eq. (2.31) becomes: The kinetic-energy term is evaluated as follows: Heat in the amount of 60 kJ must be removed per kilogram of air compressed. Example 2.14: Water at 90°C is pumped from a storage tank at a rate of 3 L/s. The motor for the pump supplies work at a rate of 1.5 kJ/s. Then water goes through a heat exchanger, giving up heat at a rate of 670 kJ/s, and is delivered to a second storage tank at an elevation 15 m above the first tank. At 90°C the density of water is 0.965 kg/L .What is the temperature of the water delivered to the second tank? Solution: The initial and final velocities of water in the storage tanks are negligible. All remaining terms are expressed in units of kJ/kg. The mass flow rate is: 𝑚 = 𝑞 𝜌 = 3 × 0.965 = 2.895 𝑘𝑔/𝑠 For the heat exchanger, 𝑄 = −670/2.895 = −231.4 𝑘𝐽/𝑘𝑔 For the shaft work of the pump, 𝑊𝑠 = 1.5/2.895 = 0.52 𝑘𝐽/𝑘𝑔 If g is 9.8 m/s2, the potential-energy term is: ∆𝐸𝑝 = 𝑔𝛥𝑧 = 9.8 × 15 = 147 𝑚2/𝑠2 = 147 𝐽/𝑘𝑔 = 0.147 𝑘𝐽/𝑘𝑔 In steady-flow process, Eq. (2.31) applies: 𝛥𝐻 = 𝑄 + 𝑊𝑠 − ∆𝐸𝑝 = −231.4 + 0.52 − 0.15 = −231.03 𝑘𝐽/𝑘𝑔 The steamtable value (Page 686) for the enthalpy of liquid water at 90 °C is: 𝐻1 = 376.9 𝑘𝐽/𝑘𝑔 𝛥𝐻 = 𝐻2 − 𝐻1 = 𝐻2 − 376.9 = −231.0 𝐻2 = 376.9 − 231.0 = 145.9 𝑘𝐽/𝑘𝑔 The temperature of water having this enthalpy is found from the steam tables (Page 685): 𝑇 = 34.83 °𝐶 Ws and ∆𝐸𝑝 are small compared with Q, and for practical purposes could be neglected. تم استخراج درجة الحرارة من جداول البخار من قيم االنثالبة واذا لم تتوفر الجدول ممكن استخدام العالقة التالية:تنويه 𝑑𝐻 = 𝐶𝑝 𝑑𝑇 Example 2.15: A steam turbine operates adiabatically with a power output of 4000 kW. Steam enters the turbine at 2100 kPa and 475 °C. The exhaust is saturated steam at 10 kPa that enters a condenser, where it is condensed and cooled to 30 °C. a) What is the mass flow rate of the steam? b) What rate must cooling water (C.W.) be supplied to the condenser, if the water enters at 15°C and is heated to 25 °C? Solution H1 C.W. H3 H2 (a) The enthalpies of entering and exiting steam from the turbine are found from the steam tables: 𝑘𝐽 Super Steam 2100 kPa and 475 °C 𝐻1 = 3411.3 (𝑃𝑎𝑔𝑒 707) 𝑘𝑔 𝑘𝐽 saturated steam at 10 kPa 𝐻2 = 2584.8 (𝑃𝑎𝑔𝑒 685) 𝑘𝑔 Kinetic-energy and potential-energy changes are negligible, and for adiabatic operation Q = 0. Eq. becomes simply 𝑄 ± 𝑊𝑠 = ∆ 𝐻 + Ė 𝑘 + ∆ Ė 𝑝 (2.31 ) 𝑊𝑠 = 𝑚 𝛥𝐻 (b) The enthalpies of the condenser are Conditions Turbine Steam C.W. Enthalpy kJ/kg. Page Saturated steam at 10 kPa 𝐻2 = 2584.8 685 Subcooled water at 30 °C H3 = 125.7 685 Entering C.W. at 15 °C 𝐻𝐶.𝑊.𝑖𝑛 = 62.9 684 Leaving C.W. at 25 °C 𝐻𝐶.𝑊. 𝑜𝑢𝑡 = 104.8 684 Equation (2.29) here reduces to 𝑚 𝑠𝑡𝑒𝑎𝑚 (𝐻3 − 𝐻2) + 𝑚 𝐶.𝑊 (𝐻𝐶.𝑊.𝑜𝑢𝑡 − 𝐻𝐶.𝑊.𝑖𝑛) = 0 4.840 (125.7 − 2584.8) + 𝑚 𝑤𝑎𝑡𝑒𝑟 (104.8 − 62.9) = 0 𝑚 𝑤𝑎𝑡𝑒𝑟 = 284.1 𝐾𝑔/𝑠 Problem 2.28. Nitrogen flows (M = 28 g/mol) at steady state through a horizontal, insulated pipe with inside diameter of 1.5 in. A pressure drop results from flow through a partially opened valve. upstream from the valve the pressure is 100 psia, 120 °F, and the velocity of 20 ft/s. If the pressure downstream from the valve is 20 psia, what is the temperature? Assume for Nitrogen that PV/T is constant, CV = (5/2) R, and CP = (7/2) R. where R = 3.407 ft lbf/(mol R) 0 = 𝑅 (𝑇2 − 𝑇1) + [( ) − 1] 𝑀 2 2 𝑃2 𝑇1 𝑃1 = 100 𝑃𝑠𝑖𝑎 𝑃2 = 20 𝑃𝑠𝑖𝑎 𝑇1 = 120 + 460.67 = 579.67 𝑅 𝑢1 = 20 𝑓𝑡/𝑠 7 0 = 3.407 (𝑇2 − 579.67) + 2 202 𝑇2 × 100 2 [( 20 × 579.67 2 ) − 1] 28 0 = (11.9 𝑇2 − 6912.2) + 5600[𝑇227.43 × 10−5 − 1] 0 = 11.9 𝑇2 − 6912.2 + 0.416 𝑇22 − 5600 0 = −12512.2 + 11.9 𝑇2 + 0.416 𝑇22 اما تحل بالدستور او بالتجربة والخطا النها معادلة من الدرجة الثانية Assume T2 = 575 R with trial and error: Ans T2 = 578.9 R Problem 2.16. Carbon dioxide gas (M = 44 g/mol) enters a compressor at conditions P1 = 15 psia and T1 = 50 °F, and is discharged at conditions P2 = 520 psia and T2 = 200 °F. The entering CO2 flows through a 4-inch-diameter pipe with a velocity of 20 ft/s, and is discharged through a 1inch-diameter pipe. The shaft work supplied to the compressor is 5360 Btu/lbmole. What is the heat-transfer rate from the compressor in Btu/h? H1 = 307 Btu/lbm V1 = 9.25 ft3/ lbm H2 = 330 Btu/lbm V2 = 0.28 ft3/ lbm Solution 𝑢𝐴 ṁ=𝑢𝐴𝜌= 𝑉 𝑚 𝜋𝐷12/4 𝑢1 = 𝑉1 = 679.26 𝑙𝑏𝑚/ℎ Δ(ρuA)fs = 0 𝑉2 𝑢2 = 𝑚 𝜋𝐷2/4 = 9.686 𝑓𝑡/𝑠 2 𝑄 − 𝑊𝑠 = ∆𝐻 + Ė𝒌 + Ė𝒑 An open system (𝐵𝑡𝑢/𝑙𝑏𝑚) (2) 𝑢22 − 𝑢12 𝑊𝑠 𝑄 = (𝐻2 − 𝐻1) + − 2 𝑀 9.6862 − 202 𝑄 = (330 − 307) + 2 − 𝑄 = 𝑚 𝑄 = 679.26 (−98.82) = −67128 𝐵𝑡𝑢/ℎ Summary A closed system ±𝑄 ± 𝑊 = ∆𝑈 + Ė𝑘 + Ė𝑝 (2.2) An open system ± 𝑄 ± 𝑊𝑠 = ∆𝐻 + Ė𝑘 + Ė𝑝 (2.31) ĖP = 0 if there is no appreciable vertical separation between the inlet –Q –W +Q +W ĖK = 0 if velocity changes is slightly. W and W = 0 If there are no moving parts (such as a pump or System Surrounding and outlet ports. s turbine). Reversible Process WIrreversible = η Wrev Enthalpy 𝑑𝐻 = 𝑑𝑈 + 𝑑(𝑃𝑉 ) Heat Capacity (5.31) 𝑑𝑈 = 𝐶𝑣 𝑑𝑇 𝑎𝑛𝑑 𝐶𝑝 = 𝐶𝑣 + 𝑅 𝐺𝑎𝑠𝑒𝑠 𝐶𝑝 = 𝐶𝑣 = 𝐶 𝐿𝑖𝑞𝑢𝑖𝑑𝑠 (2.13) 𝑑𝐻 = 𝐶𝑝 𝑑𝑇 Flow in Open systems 𝑑𝑚𝐶𝑉 Unsteady state Steady state + Δ(𝜌𝑢𝐴)𝑓𝑠 = 0 (2.25) 𝑑𝑡 ∑ ṁin − ∑ ṁout = 0 Δ(ρuA)fs = 0 𝑢𝐴 ṁ=𝑞𝜌=𝑢𝐴𝜌= (2.23𝑎) 𝑉