Chemistry: Introduction, Scientific Method, & Conservation of Mass
advertisement

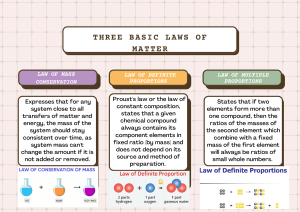
C1501 Introduction • Chemistry: the branch of science that describes matter; it’s chemical and physical properties, the chemical and physical changes it undergoes and the energy changes that accompany these processes. • Matter: anything that has mass and occupies space. • Atoms – are the smallest units that we associate with the chemical behavior of matter ; atoms make up matter. • Mass – is a measure of the quantity of matter that an object contains; mass does not vary with location, i.e. moon or earth object has same mass. • Chemistry can be described as a central science. It relies on the foundations of mathematics and physics, and in turn underlies the life sciences i.e. biology and medicine. To understand living systems fully, we need to first understand the chemical reactions and chemical influences that operate within them. Introduction • In our search for understanding, we eventually have to ask fundamental questions such as: 1. How do substances combine to form other substances? How are energy changes involved in chemical and physical changes? 2. How is matter structured? How are atoms and the ways that they combine related to the properties of the matter that we can measure, such as colour, hardness, chemical reactivity and electrical conductivity? 3. What fundamental factors influence the stability of a substance? How can we force a desired but energetically unfavourable change to take place? What factors control the rate at which a chemical change takes place? The Discovery of Cisplatin The Discovery of Cisplatin • In 1964 Barnet Rosenberg and his co-workers at Michigan State University were studying the effects of electricity on bacterial growth. They inserted platinum wire electrodes into a live bacterial culture and allowed an electric current to pass. • After 1 or 2 hours, they noted that the cell division in the bacteria had stopped. Further experiments showed that cell division was inhibited by a substance containing platinum, produced from the platinum electrodes by the electric current. The researchers realised that a substance such as this one could be useful as an anticancer drug, because cancer involves uncontrolled cell division. • Later, research confirmed this view, and today the platinum-containing substance cisplatin is a leading anticancer drug. The Discovery of Cisplatin • This story illustrates 3 significant reasons to study chemistry: 1. Chemistry has important practical applications. 2. Chemistry is an intellectual enterprise, a way of explaining our material world. 3. Chemistry figures prominently in other fields. The Scientific Method • Science is a frame work of gaining and organizing knowledge; it is not simply a set of facts, but also a plan of action i.e. a procedure for processing and understanding certain types of information. The process that lies at the centre of scientific enquiry is called the scientific method. • There are many scientific methods depending on the nature of the specific problem under study and on the particular investigator involved. However, below is a useful general framework for a generic scientific method: The Scientific Method Steps in the scientific method 1. Making Observations Observations may be qualitative, e.g the sky is blue, water is a liquid, etc. Observations may also be quatitative, e.g water boils at 100°C, 10.0mL of water weighs 10.0 g, etc. A qualitative observation does not involve a number. A quantitative observation(Called a measurement) involves both a number and a unit. 2. Formulating Hypothesis A hypothesis is a possible explanation for the observation. The scientific Method 3. Making Predictions The hypothesis is then used to make predictions that can be tested by performing an experiment. 4. Performing Experiments An experiment is carried out to test the hypothesis. This involves gathering new information that enables the investigator to decide whether the hypothesis is correct i.e. whether it is supported by the new information learned from the experiment. Experiments always yield new observations, and this brings the process back to the beginning. The Scientific Method • An experiment can be defined as an observation of natural phenomena carried out In a controlled manner so that results can be duplicated and rational conclusions obtained. • As scientist observe nature, they often see that the same observation applies to many different systems. Such generally observed behaviour is formulated into a statement called a natural law (or just simply a law). A law is a concise statement or mathematical equation about a fundamental relationship or regularity of nature. • For example, the observation that there is no observable change is the quantity of matter during a chemical reaction or during a physical change is known as the law of conservation of matter/mass. The Scientific Method • The law tells us what happens, but it offers no explanation for why we observe a certain phenomenon. To try to explain why, we continue to make observations, formulate hypothesis and test these against observations. • If a hypothesis successfully passes many tests it becomes known as a theory. A theory is a tested explanation of basic natural phenomenon. experiments results hypothesis further exps negative results positive results theory further exps 12 Law of Conservation of Mass • The French chemist, Antoine Lavoisier (1743-1794) was one of the first chemists to insist on the use of the balance in chemical research. By weighing substances before and after a reaction, he demonstrated the Law of conservation of Mass. This law states that the total mass remains constant during a chemical reaction. • Lavoisier conducted a series of combustion reactions and showed that when a material burns, a component of air (which he called oxygen) combines chemically with the material. E.g. when mercury is heated in air, it burns or combines with oxygen to give a red-orange substance whose modern name is mercury(II) oxide. We can represent the chemical reactions as follows: Law of Conservation of Mass Mercury + oxygen mercury (II) oxide The arrow in the above representation means “changes to.” • By strongly heating the red-orange substance, Lavoisier was able to decompose it to yield the original mercury and oxygen gas. • Therefore the law of conservation of mass can be represented as follows: MASSReactants = MASSProducts Law of Conservation of Mass Example 1 a) You heat 2.53 grams of metallic mercury in air, which produces 2.73 grams of a red-orange residue. Assume that the chemical change is the reaction of the metal with oxygen in the air. Mercury + oxygen red-orange residue What is the mass of oxygen that reacts? b) When you strongly heat the red-orange residue, it decomposed to give back the mercury and releases the oxygen, which you collect. What is the mass of oxygen you collect? Law of Conservation of Mass Solution 1 Strategy: Apply the law of conservation of mass to the reactions. MASSReactants = MASSProducts a) From the law of conservation of mass, Mass of mercury + mass of oxygen = mass of red-orange residue 2.53 g + mass of oxygen = 2.73 g Mass of oxygen = 2.73g – 2.53g = 0.20 grams Therefore the mass of oxygen that reacts is 0.20 grams Law of Conservation of Mass b) When the red-orange residue is strongly heated, the mass of oxygen collected is 0.20 grams. Law of Conservation of Mass • Definition of Terms Mass: the quantity of matter in a material. It remains the same regardless of where you are. Weight: The force exerted on a body by gravity. It changes as gravitational acceleration changes e.g. on earth vs on the moon. Fg = mg Fg – gravitational force m – mass Law of Conservation of Mass Example 2 Methane gas burns in oxygen according to the reaction: CH4(g) + 2O2(g) CO2(g) + 2H2O(l) Reactants : Methane gas (CH4) Oxygen gas (O2) Products: Carbon dioxide gas(CO2) Water (H2O) Note: subscripts tell how many of a particular type of atom are inside a molecule. E.g. there are 4 hydrogen atoms in a molecule of methane and 2 hydrogen atoms in a molecule of water. Co-efficients tell how many of each particle is involved in the reaction. E.g. 2 molecules of oxygen react with 1 molecule of methane. Law of Conservation of Mass a) How would you draw this reaction as particles and show conservation of mass? Solution: Key: =C =H =O + + Law of Conservation of Mass Note about showing “conservation“ in particle diagram: If you have the reaction: A2 + B2 A3B You should show conservation by drawing: + i.e. 3A2 + B2 2A3B Do not simply add stray “atoms” to molecules. It changes them into different substances. Law of Conservation of Mass b) If 16 grams of CH4 reacts completely with 64 grams of O2, what mass of products should form? Solution: CH4(g) + 2O2(g) CO2(g) + 2H2O(l) 16 g 64 g Xg MASSReactants = MASSProducts ∴ (mass of methane + mass of oxygen) = mass of carbon dioxide + mass of water) Substituting: Mass products = 16g + 64g = 80g Law of Conservation of Mass c) If 32g of CH4 reacts completely with 128g of O2, and 88g of CO2 forms, how many grams of H2O form? CH4(g) + 2O2(g) CO2(g) + 2H2O(l) mass of methane + mass of oxygen = mass of carbon dioxide + mass of water Substituting: 32g + 128g = 88g + mass of water Mass of water = (32g + 128g) – 88g = 72g Law of Conservation of Mass d) If 8g of CH4 reacts completely with oxygen, and 22g of CO2 and 9g H2O form, how much oxygen was consumed? Solution: CH4(g) + 2O2(g) CO2(g) + 2H2O(l) (mass of methane + mass of oxygen) = (mass of carbon dioxide + mass of water) Mass of oxygen= (mass of carbon dioxide + mass of water) – mass of methane = (22g + 9g) – 8g = 31g – 8g = 23g Law of Conservation of Mass e) 4 g of CH4 reacts with 20 g O2. The CH4 is used up completely, but there is some O2 left over. Given that 20g of products was formed, how much oxygen was used up? Law of Conservation of Mass Solution: CH4(g) + 2O2(g) CO2(g) + 2H2O(l) 4g Xg 20g X= 16g was used up. Since 20g of oxygen was available, the left over oxygen would be; = 20 g available O2 – 16 g used up O2 = 4g left over Phases and Classification of Matter Learning objectives/skills to develop 1. Describe the basic properties of each physical state of matter: solid, liquid and gas. 2. Define and give examples of atoms and molecules. 3. Classify matter as an element, compound, homogeneous mixture or heterogeneous mixture with regards to its physical state and composition. 4. Explain the difference between pure substances and mixtures. Phases and Classification of Matter • There are two principal ways of classifying matter: – By its physical state as a solid, liquid, or gas which is dependent on the conditions, i.e. temperature, pressure. – By its chemical constitution as an element, compound, or mixture. Phases/States of Matter: Solids, Liquids, and Gases • Solid: the form of matter characterized by rigidity; a solid is relatively incompressible and has a fixed shape and volume. • Liquid: the form of matter that is a relatively incompressible fluid; liquid has a fixed volume but no fixed shape. • Gas: the form of matter that is an easily compressible fluid; a given quantity of gas will fit into a container of almost any size and shape. Phases and Classification of Matter: Elements, Compounds and Mixtures • Matter can be classified into two categories: Pure substances and Mixtures. • Pure substances that can not be broken down into simpler substances by chemical changes are called elements. Iron, silver, gold, aluminium, oxygen, hydrogen, carbon, copper and sulphur are examples of common elements we encounter. There are 113 elements in the periodic table of the elements; about 90 occur naturally and 2 dozen or so have been created in laboratories. The smallest unit of an element is the atom. • Pure substances that can be broken down into simpler substances by chemical changes are called compounds. A compound is, therefore, a substance composed of two or more elements chemically combined (e.g. NaCl, H2O). This breakdown may produce either elements or other compounds or both. E.g. mercury (II) oxide can be decomposed to yield mercury and oxygen. The smallest unit of a compound is the molecule. Phases and Classification of Matter: Elements, Compounds and Mixtures • The properties of combined elements are different from those of free elements. For example, white crystalline sugar(sucrose) is a compound resulting from the chemical combination of the element carbon, which is a black solid in one of its uncombined forms, and two elements oxygen and hydrogen, which are colourless gases when uncombined. • A mixture is a material that is composed of two or more types of matter that can be separated by physical changes/means such as distillation. Unlike a pure compound, a mixture has variable compositions. When you dissolve sodium chloride in water, you obtain a mixture, its composition depends on the relative amount of sodium chloride dissolved. You can separate this mixture into solid sodium chloride and water by distillation. Phases and Classification of Matter: Elements, Compounds and Mixtures Mixtures can be classified into two types: 1. Homogeneous mixtures 2. Heterogeneous mixtures A homogeneous mixture, also known as a solution is mixture that is uniform in its properties throughout given samples. When sodium chloride is dissolved in water, you obtain a homogeneous mixture. Air is a homogeneous gaseous mixture of primarily nitrogen and oxygen gases. The gases are physically combined but not chemically combined. Phases and Classification of Matter: Elements, Compounds and Mixtures • A heterogeneous mixture is a mixture that consists of physically distinct parts, each with different properties. E.g. a mixture of sand and water, a mixture of oil and water, fruit salad, a mixture of potassium dichromate power and iron fillings, a mixture of salt and sugar mixed together. • A phase is one of several different homogeneous materials present in a portion of matter under study. E.g. a mixture of ice cubes in water is said to be composed of two phases: one phase is ice(solid) and the other is liquid water. Ice floating in a solution sodium chloride in water consist of two phases: ice and the liquid solution. Note that a phase can be a pure substance in a particular state or a solution in a particular state (solid, liquid or gaseous). Phases and Classification of Matter Law of Definite Proportions • Antoine Lavoisier’s quantitative experiments showed that combustion involved oxygen. He also discovered that life was supported by a process that also involved oxygen and was similar in many ways to combustion. • After 1800, chemistry was dominated by scientists who, following Lavoisier’s example, performed careful weighing experiments to study the course of chemical reactions and to determine the composition of various chemical compounds. • One of these chemists, a French, Joseph Proust (1754-1826) showed that a given compound always contains exactly the same proportion of elements by mass. This principle of the constant composition of compounds, originally called Proust’s Law, is now known as the Law of Definite Proportions. – The law of definite proportions states that a pure compound, whatever its source, always contains definite or constant proportions of the elements by mass; unique composition for each compound but always the same for a particular compound. • E.g. copper carbonate is always 5.3 parts copper to 4 parts oxygen to 1 part carbon (by mass); and sodium chloride always has 1 part sodium & 1.54 parts chlorine. Law of Definite Proportions • One of these chemists, a French, Joseph Proust (1754-1826) showed that a given compound always contains exactly the same proportion of elements by mass. This principle of the constant composition of compounds, originally called Proust’s Law, is now known as the Law of Definite Proportions. – The law of definite proportions states that a pure compound, whatever its source, always contains definite or constant proportions of the elements by mass; unique composition for each compound but always the same for a particular compound. • E.g. copper carbonate is always 5.3 parts copper to 4 parts oxygen to 1 part carbon (by mass); and sodium chloride always has 1 part sodium & 1.54 parts chlorine. Implications of the law of Definite Proportions • Constant composition implies constant properties (e.g.water always boils at 100°C and freezes at 0°C). Law of Multiple Proportions • Proust’s discovery stimulated John Dalton (1766-1844), an English school teacher, to think about atoms. Dalton reasoned that if elements were composed of tiny individual particles, a given compound should always contain the same combination of these atoms. This concept explained why the same relative masses of elements were always found in a given compound. • But Dalton discovered another principle that convinced him even more of the existence of atoms. He noted, for example, that carbon and oxygen form two different compounds that contain different relative amounts of carbon and oxygen: Law of Multiple Proportions Mass of oxygen that combines with 1g carbon Compound I 1.33 g Compound II 2.66 g Dalton noted that compound II contains twice as much oxygen per gram of carbon as compound I, a fact that could be easily explained in terms atoms. Compound I might be CO and compound II CO2. This principle, which was found to apply to compounds of other elements as well, became known as the Law of Multiple proportions: When two elements form a series of compounds, the ratios of the masses of the second element can always be reduced to small whole numbers. Law of Multiple Proportions • Compounds of differing mass ratios of the same elements are found, but they will have different properties. E.g. CO, CO2 H2O, H2O2 NO, NO2 Example 1. Given the following date for 3 compounds composed of nitrogen and oxygen, propose possible formulas for compounds I, II and III: Law of Multiple Proportions Compound I Compound II Compound III Solution: 𝐼 1.750 2 = = 𝐼𝐼 0.8750 1 𝐼𝐼 0.8750 2 = = 𝐼𝐼𝐼 0.4375 1 𝐼 1.750 4 = = 𝐼𝐼𝐼 0.4375 1 Mass of Nitrogen that combines with 1g of Oxygen 1.750 g 0.8750 g 0.4375 g Law of Multiple Proportions • Compound I contains twice as much nitrogen (N) per gram of oxygen (O) as does compound II. • Compound II contains twice as much nitrogen per gram of oxygen as does compound III. These data can be summarised by the following sets of formulas: Compound I N2O NO N 4O2 Compound II NO or NO 2 N2O2 Compound III NO2 NO4 N204 Law of Multiple Proportions 2. Hydrogen and Oxygen are known to form form 2 compounds. The hydrogen content in one is 5.93% and that of the other is 11.2%. Show that this data illustrates the law of multiple proportions. Solution: In the first compound: Hydrogen = 5.93% Oxygen = (100-5.93)% = 94.07% In the first compound the number of parts of oxygen that combine with 94.07 one part by mass of hydrogen = = 15.86 parts 5.73 Law of Multiple Proportions In the second compound: Hydrogen = 11.2% Oxygen = (100-11.2)%=88.88% In the second compound the number of parts by mass of oxygen that 88.88 combine with one part by mass of hydrogen = =7.9 11.2 Ratio of the masses of oxygen that combine with fixed mass of hydrogen is 15.86 : 7.9 or 2: 1. This is consistent with the law of multiple proportions.