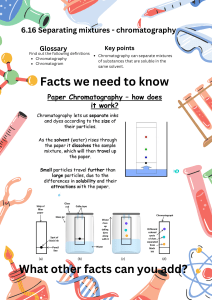
Santa Monica College Chemistry 11 Paper Chromatography: Separation and Identification of Five Metal Cations Objectives Known and unknown solutions of the metal ions Ag +, Fe3+, Co2+, Cu2+ and Hg2+ will be analyzed using paper chromatography. An unknown solution containing some of these cations will be identified by comparison to the Rf values and colors of the stained spots of known solutions. Background Most chemists and many other scientists must routinely separate mixtures and identify their components. The ability to qualitatively identify the substances found in a sample can be critical. For example, an environmental chemist investigating samples of polluted ground water will want to know which toxic ions might be present in a sample. Chromatography is one of the first tools employed in such situations. Using this technique many types of mixtures can be separated into the component pure substances, and by comparison to a standard sample, each component substance can also be tentatively identified. Many varieties of chromatography exist, each one designed to separate specific types of mixtures. The common feature of each type of chromatography is that a mobile phase (a liquid or gas) is pushed through a stationary phase (a solid). Component B Component A Stationary Phase with Eluent Figure 1. A typical column chromatography experiment demonstrates the separation of a two-component mixture. Paper Chromatography To illustrate the technique, consider the separation of a twocomponent mixture using Column Chromatography, shown in Figure 1. The column is packed with a solid material which is the stationary phase. A liquid solvent or eluting solution – the mobile phase – is poured into the column and completely wets the solid packing material. Then the mixture is loaded onto the top of the wet column and more of the eluting solution (or eluent) is added. Gravity pulls the mobile phase down through the stationary phase and the components in the mixture start to move through the column at different rates. In Figure 1, Component A moves faster than Component B; thus Component B is retained on the column for a longer time than Component A. Usually this is due to a difference in solubility of the two compounds in the solvent, and/or to a difference in attraction to the solid packing material. As the eluting solution continues to be added to the column, eventually the two components will exit the column separately. The time taken for each component to exit the column is called its retention time. Retention time will be reproducible for each component under the given set conditions – mobile and stationary phase identities, temperature and column width. Page 1 of 6 Santa Monica College Chemistry 11 Once the components exit the column, the solvent can be removed by evaporation and the pure components can be further analyzed. Tentative identification of the components can be achieved by comparing the unknown mixture with a carefully prepared known mixture. If a known component has the same retention time as an unknown component under the same conditions, it is probable – but not conclusive – that the two components are the same. Further analysis may be needed to confirm this hypothesis. If the known and the unknown samples have different retention times, then it is very unlikely that the two components are identical. Figure 2: Thin-layer chromatography of black ink after development. This picture demonstrates a common problem where the spots widen as they move up the plate, eventually merging at the top of the plate. Other types of chromatography might use capillary action – the attraction of a liquid to a solid surface – to pull a solvent through solid material. Figure 2 shows the results of a Thin-Layer Chromatography experiment. The stationary phase is piece of plastic which is coated with a powdered solid. A liquid solvent has been passed through two black ink spots on the solid surface, pulled through the stationary phase by capillary action. The results show that the black ink is actually a mixture containing several different colored substances. Each component of the ink has a slightly different solubility in the mobile phase, so that when the liquid is pulled through the stationary phase each component moves at a different rate, separating the ink into spots of different colors. Paper Chromatography works in a similar manner, using paper as the stationary phase. An everyday occurrence of paper chromatography can be observed when an ink-written page comes in contact with water. The ink runs and several colors are separated in the ink streak. The table below lists several varieties of chromatography and typical identities of the phases. Type of Chromatography Mobile Phase Stationary Phase Gas (GC) inert gas (helium) waxy liquid or silicone inside narrow tubing Liquid (LC, HPLC, column) solvent/solvent mixture (organic or aqueous) solid packing (silica, alumina) Paper solvent/solvent mixture (organic or aqueous) paper Thin-Layer (TLC) solvent/solvent mixture (organic or aqueous) silica/alumina coated glass, plastic or metal Paper Chromatography Page 2 of 6 Santa Monica College Chemistry 11 In this experiment, a mixture of 5 metal cations will be separated using Paper Chromatography. The metal ions – Ag+1, Fe+3, Co+2, Cu+2, and Hg+2 – have differing solubility in the mobile phase (an aqueous solution of HCl with ethyl and butyl alcohol) and will move at different rates up the paper. The different metal ion solubilities are probably due to the formation of various compounds with the chloride ion and their varying ability to dissolve in the organic solvent. A diagram showing how to prepare the paper (a chromatograph) is shown in Figure 3. Standard solutions containing each individual ion will be spotted onto the paper using a capillary tube, along with a standard solution containing a mixture of all five ions. An unknown will also be spotted onto the paper. Once the paper is prepared, it will be developed by placing the paper into the eluent. After 75-90 minutes, the results can be visualized by wetting the paper with an aqueous solution containing potassium iodide (KI) and potassium ferrocyanide (K 4[Fe(CN)6]). The unique color observed for each ion is produced by a chemical reaction with the visualization solution. This is one useful way to identify which ions are present in an unknown mixture. 20 cm Your Name(s) Tape 12 cm (5 single ions, 1X each) mixt. (3X) (2X and 4x, same unknown) Known ions 1.5 cm Unknown Mixture Figure 3: Preparation of the paper for the chromatography experiment The distance the ion moves up the paper can also be used to identify the ion. However, since students will develop their chromatographs for different amounts of time and under slightly different conditions, each student will obtain slightly different measured distances for a given ion. The ratio of the distance moved by an ion (D) to the distance moved by the solvent front (F) is characteristic and should be nearly the same for all students. This ratio is called the retention factor, Rf, where: Rf = Paper Chromatography D F Page 3 of 6 Santa Monica College Chemistry 11 Procedure Safety Avoid contact with the metal ion solutions, the eluting solvent, and the visualizing solution. Use disposable gloves to touch your chromatogram after the elution occurs, and for the remainder of the experiment. Do not breathe the vapors of the eluting solvent or the visualizing solution. Place the wet chromatogram on a paper towel, not directly on the laboratory bench. Use the visualizing solution only in the area of the lab designated by your instructor. Dispose of your gloves and chromatogram in the specified waste container after the experiment is finished. Wash your hands thoroughly after contact with all solutions in this lab. Materials and Equipment Chemicals: 0.1 M aqueous solutions of AgNO3, Hg(NO3)2, Fe(NO3)3, Co(NO3)2 and Cu(NO3)2, each with dedicated capillary tubes; eluting solution (aqueous HCl with ethyl and butyl alcohol); visualizing solution (aqueous solution of KI and K4[Fe(CN)6]). Equipment: Clean piece of chromatography paper, disposable Latex gloves (nitrile gloves are available in the stockroom for people with allergies to Latex), 600-mL beaker, plastic wrap, forceps or beaker tongs, ruler Preparation of the Paper for Chromatography 1. Obtain a piece of filter paper with the dimensions shown in Figure 3. Make sure the paper is clean and without tears or folds. Use a pencil (not a pen!) and a ruler to draw a line across the paper 1-cm from the long edge of the paper. Spot the metal ion solutions on this line. Write your name in pencil in the upper left-hand corner of the paper. 2. Practice spotting water and/or ion solutions onto a strip of filter paper so that you know how to create spots of the correct size. Use glass capillary tubes to spot the ions onto the paper. A solution is applied by lightly and quickly touching a capillary tube containing the solution to the line you drew on the paper. The spots should be 5 – 8 mm in diameter. Spots larger than this will excessively spread out during the experiment and make analysis difficult. 3. Known 0.1 M aqueous solutions of AgNO3, Hg(NO3)2, Fe(NO3)3, Co(NO3)2, and Cu(NO3)2 are provided in test tubes, each containing two or three capillary tubes. Starting on the left, mark the identity of the ion underneath each spot with a pencil; then spot each known ion carefully onto the line. Be careful to avoid contaminating the capillary tube with other ions and replace the capillary tubes back into the correct test tube. A test tube containing a known mixture of all five ions is also provided with a set of capillary tubes. Spot this mixture onto the line as well. Because this solution is more dilute than the single-ion known solutions, apply the known mixture three times, letting the spot dry between each application. A heat lamp will help to dry the spot more quickly. 4. Several unknowns are also provided in test tubes, along with capillary tubes. Your instructor will tell you which unknown should be used. The unknowns will contain between one and four cations, and are more dilute than the single-ion known solutions. The unknown will also need to be applied two and four times for the two trials, letting the spot dry between each application. In case of error, you should spot the unknown in two places along the line so that two trials are available for analysis. Paper Chromatography Page 4 of 6 Santa Monica College Chemistry 11 Developing the Chromatography Paper 5. Place a piece of tape along the upper right edge, as shown in Figure 3. Then form a cylinder by connecting the two short edges of the paper with the tape. Make sure the edges do not touch. The paper should look similar to Figure 4 below. Figure 4. Folded paper should look like this prior to developing the experiment. 6. Obtain ~ 15-mL of the eluting solution. Carefully pour some of this solvent into a 600-mL beaker and carefully swirl for a second or two. Caution: Do not breathe the vapors from this solution! Make sure that the level of the liquid will be below the spot line on the paper once the paper is placed in the developing chamber. 7. Place the paper cylinder into the beaker with the marked edge down. The spots should be above the level of the solvent. The paper should not be touching the sides of the beaker. Carefully cover the beaker with plastic wrap and place it in the hood for 75-90 minutes. The solvent should start to move up the paper. Once the beaker is covered, make sure it is level and do not disturb it during the development period. Your instructor may lecture or have an assignment for you to work on while you wait. Visualization and Analysis of the Paper 8. Once the development period is over, wear disposable gloves and remove the paper from the beaker. Latex gloves are available in the lab and nitrile gloves are available in the stockroom for people with Latex allergies. Let any solvent drip back into the beaker, then remove the tape. Lay the chromatography paper on a paper towel and immediately mark the solvent front with a pencil. Pour the used eluting solvent into the waste container provided. Dry the paper under a heat lamp in the hood. Caution: Do not breathe the vapors! Also be careful not to burn the paper under the lamp. 9. Once the paper is dry, bring it to the visualization station on the paper towel. Briefly dip the paper into the visualizing solution located in a shallow dish in the fume hood. Lift the paper out of the solution immediately and let any excess drip off at the station. Place the wet paper onto a dry paper towel and dry it under a heat lamp immediately, then carry it to your bench for analysis. 10. Find each known single-ion first and record the colors you observe. Some spots may fade over time, so record the colors while the paper is still wet. Measure the distance each spot moved (D) with a ruler. Make your measurements to the center of each spot, as shown in Figure 5 on the next page. Record these measurements in the data table on your Lab Report. Paper Chromatography Page 5 of 6 Santa Monica College Chemistry 11 11. Measure the distance to the solvent front (F), as shown below in Figure 5. The value of F should be approximately the same across the entire paper. Record your measurements in the data table on your Lab Report. Then use your values of D and F to calculate the retention factor, Rf, for each ion. Each observed spot will have its own Rf -value. Solvent Front F D Spotting Line Figure 5. Measurement of distances used in the calculation of Rf for a spot. 12. In the lane containing the mixture, find each ion and record the distance moved by each ion. Calculate the Rf for each ion in this lane. The values should closely match those observed in the single-ion knowns. 13. In the lane containing the unknowns, locate the center of each spot observed, record its distance, and calculate the Rf values. Use the lane that has the clearest spots. The color and Rf values for the unknown spots should closely match some of the known ions. You should now be able to identify which ion or ions are found in your unknown. Record your data in the corresponding table on your Lab Report. 14. Make a sketch of your chromatogram in the space provided on your Lab Report, being sure to indicate the position and approximate size and shape of each spot on the paper. Clean-Up Place your chromatograph and the used gloves in the waste container provided. The used eluting solution should already have been placed into another waste container. Note that two different waste containers are provided for this experiment, so be sure to read the labels and use the correct one! Finally, be sure to wash your hands thoroughly before leaving the laboratory. Paper Chromatography Page 6 of 6