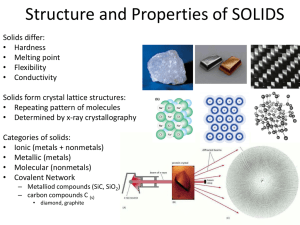
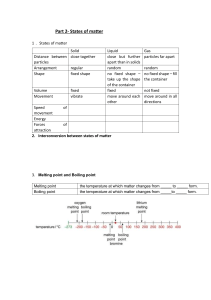
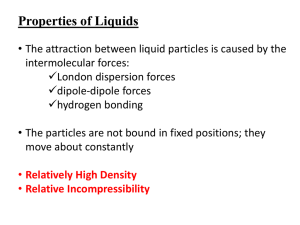
Properties of Solids • Mechanical Properties – Rigidity: Solids retain their shape and resist deformation. – Hardness: The resistance to scratching or indentation. – Brittleness vs. Malleability: Brittle solids break easily, while malleable solids can be shaped. • Thermal Properties –Melting Point: The temperature at which a solid becomes a liquid. –Thermal Expansion: Solids expand on heating due to increased vibration of particles. • Electrical Properties –Conductivity: Depends on the type of bonding (e.g., metallic solids are good conductors; covalent network solids are insulators). • Optical Properties – Transparency vs. Opacity: Some solids allow light to pass through, while others block it. – Refractive Index: The measure of how much light bends when passing through the solid. • Density – Solids are generally denser than liquids and gases due to closely packed particles. Types of Solids Crystalline Solids • Definition: Solids in which particles (atoms, ions, or molecules) are arranged in a regular, repeating pattern.Examples: Salt (NaCl), Diamond, Quartz.Properties:Have a definite geometric shape. • Sharp melting points (melt at a specific temperature). • Exhibit anisotropy (properties vary with direction). • High stability due to strong intermolecular forces. Amorphous Solids • Definition: Solids in which particles are not arranged in a regular pattern; they lack a long-range order. • Examples: Glass, Rubber, Plastic. • Properties:No definite geometric shape. • Gradual softening over a range of temperatures (no sharp melting point). • Isotropic (properties are the same in all directions). • Less stable and can flow over time (pseudo-solid behavior). TYPES OF CRYSTALS Ionic Crystals • Constituent Particles: Positive and negative ions held together by strong electrostatic forces (ionic bonds). Examples: Sodium chloride (NaCl), Potassium bromide (KBr), Magnesium oxide (MgO). • Properties: • Hard and Brittle: Strong ionic bonds make ionic crystals hard but also prone to shattering under force. • High Melting and Boiling Points: The strong electrostatic forces require a lot of energy to overcome. • Poor Electrical Conductivity in Solid State: Ions are fixed in place and cannot move. • Good Electrical Conductivity in Molten or Dissolved State: Ions become free to move and conduct electricity. • Solubility: Many ionic crystals dissolve in polar solvents like water. Covalent (Network) Crystals • Constituent Particles: Atoms connected by covalent bonds in a continuous network throughout the crystal. Examples: Diamond, Graphite, Silicon dioxide (Quartz), Silicon carbide (SiC). • Properties: • Extremely Hard and Strong: The rigid network of covalent bonds makes these crystals exceptionally hard (e.g., diamond is the hardest natural substance). • Very High Melting Points: Breaking covalent bonds requires a large amount of energy. • Non-conductive (Except Graphite): Electrons are localized in bonds, except in graphite where delocalized electrons enable conductivity. • Insoluble: Covalent crystals are generally insoluble in most solvents due to the strength of the bonds. Molecular Crystals • Constituent Particles: Molecules held together by weak intermolecular forces (e.g., van der Waals forces, dipole-dipole interactions, or hydrogen bonds). Examples: Ice (H₂O), Carbon dioxide (dry ice), Iodine (I₂), Sugar (C₆H₁₂O₆). • Properties: • Soft and Low Melting Points: Weak intermolecular forces are easy to overcome. • Non-conductive: Molecules do not have free electrons or ions to conduct electricity. • Volatility: Some molecular crystals (e.g., dry ice) easily sublime due to weak forces. • Solubility: Depends on the polarity of the molecules; polar molecular crystals dissolve in polar solvents, and nonpolar crystals dissolve in nonpolar solvents. Metallic Crystals • Constituent Particles: Positive metal ions surrounded by a "sea of delocalized electrons." Examples: Iron (Fe), Copper (Cu), Aluminum (Al), Gold (Au). • Properties: • Good Electrical and Thermal Conductivity: Delocalized electrons allow free flow of electric current and heat. • Malleable and Ductile: The metallic bonds are flexible, allowing the metal to be shaped without breaking. • Shiny and Lustrous: Free electrons reflect light, giving metallic crystals their shiny appearance. • Variable Melting Points: Melting points depend on the strength of the metallic bonds (e.g., tungsten has a high melting point, while mercury is liquid at room temperature). • High Density: Close packing of metal ions contributes to their high density. Gain or Loss SOLID to LIQUID LIQUID to VAPOR VAPOR to SOLID VAPOR to LIQUID