SIWES Program Report: History, Objectives, and Importance
advertisement

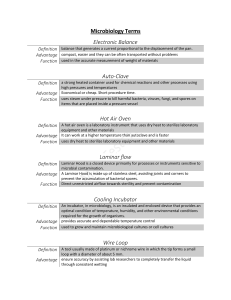
CHAPTER ONE 1.1 HISTORY AND BACKGROUND OF SIWES PROGRAM The Student Industrial Work Experience Scheme (SIWES) is a planned and supervised training intervention based on stated and specific learning and career objectives and geared towards developing the occupational competencies of the participants. It is a program required to be undertaken by all students of tertiary institutions in Nigeria pursuing courses in “specialized Engineering, Technical, Business, Applied Sciences and Applied Arts” (ITF, 2004). It is a Skill Training Program designed to prepare and expose students of the University to the Industrial Work situation they are likely to meet after graduation. The need for the establishment of the scheme aroused when there was a growing concern among industrialists that graduates of institutions of higher learning lacked adequate practical background required for employment in industries. Thus, the employers were of the opinion that the theoretical education in higher institutions was not responsive to the needs of employers of labour. In line with the foregoing, SIWES program was designed to complement classroom teaching in the course of studies and to acquaint students with the skills needed in the industries after graduation. The Student Industrial Work Experience Scheme (SIWES) was established in 1973 by the Industrial Training Fund (ITF) as one of her programs. It was designed to give Nigerian Students studying occupationally-related courses in higher institutions the experience that would supplement their theoretical learning in order to solve the problem of lack of adequate practical skills preparatory for employment in industries by graduate. SIWES is the accepted skills training program which forms part of the approved minimum academic standards in the various degree programs for all the Nigerian Universities. It is an effort to bridge the gap existing between theory and practice of engineering and technology, science, agriculture, media management and other profession educational programs in Nigerian tertiary institutions. It is aimed at exposing students to machines and equipments, professional work method and ways of safe-guarding the work areas and workers in industries and other organization. The scheme is a tripartite program involving the students, the universities and the industry (employers of labour). It is funded by the Federal Government of Nigeria and jointly coordinated by the Industrial Training Fund (ITF) and the National Universities Commission (NUC). 1 1.2 AIMS AND OBJECTIVES The aim of the SIWES Directorate is to ensure the proper training of the University students in preparing them for the world of industrial work. The Objectives are to: Provide an avenue for students in Nigerian schools to acquire industrial skill and experience in their course of study. Prepare students for the work situation they are likely to face after graduation. Expose students to work method and techniques in handling equipment and machinery that may not be available in the Universities. Provide students opportunities to apply their theoretical knowledge and acquire work practice. Make transitions from school to the world of work easier and this enhances student contact for later placement after graduation. Enlist and strengthen employer involvement in the entire educational process of prepare students for employment in Industries and Commerce. To help students on contacts for job placements. Provide students with an opportunity to apply their knowledge in real work situation thereby bridging the gap between theory and practice. 1.3 BODIES INVOLVED IN THE MANAGEMENT OF SIWES The bodies involved in the management of SIWES include; Federal Government Industrial Training Fund (I.T.F.) Other supervising agencies are; National University Commission (N.U.C.) National Board for Technical Education (N.B.T.E.) and National Council for Colleges of Education (N.C.C.E.) 1.3.1 THE FUNCTIONS OF THESE AGENCIES ABOVE INCLUDE; Ensure adequate funding of the scheme Establish SIWES and accredit SIWES unit in the approved institution 2 Formulate policies and guidelines for participating bodies and institutions as well as appointing SIWES coordinators and supporting staff Supervise students at their places of attachment and sign their log-book and I.T.F. forms Vet and process students log-books and forward same to I.T.F. area office Ensure payment of allowances for the students and supervisors. Therefore, the success or otherwise of the SIWES depends on the efficiency of the ministries, I.T.F., Institutions, Employers of labor and the general public involved in articulation and management of the program. Thus, the evaluation of SIWES in Tertiary Institutions in meeting up with the needs for the establishment of the program. 1.4 VISION STATEMENT It is the vision of the SIWES scheme to ensure that students get practical training of what they have been taught in theory so as to meet their future demand of world of work. 1.5 MISSION STATEMENT The mission of SIWES is to ensure that students on graduation from their various fields of study and having participated in the industrial training exercise will be able to handle practical demands of job and meet up with the challenges and demands of work on graduation. 1.6 IMPORTANCE OF SIWES TO MICROBIOLOGY 1. It develops and enhance personal attributes such as critical thinking, creativity, initiative, resourcefulness, leadership, time management, presentation skills and interpersonal skills. 2. It exposes students to more practical works, methods and technique 3. It provides students the opportunity to apply their theoretical knowledge in real life situations. 4. It makes students more useful for themselves and their field of work. 5. It helps students get familiar with equipment and machine used in Microbiology laboratory. 3 CHAPTER TWO THE DESCRIPTION OF ESTABLISHMENT OF ATTACHMENT 2.1 Microbiology Laboratory, Ekiti State University, Ado-Ekiti, Ekiti State The Microbiology Laboratory at Ekiti State University (EKSU) is a well-equipped facility dedicated to microbiological research, teaching, and practical training. Located within the Faculty of Science complex, the laboratory serves as a crucial center for both undergraduate and postgraduate studies in microbiology. The laboratory complex consists of several specialized sections including the general microbiology lab, molecular biology unit, media preparation room, sterilization room, and research units. The facility features adequate ventilation, emergency exits, and proper waste disposal systems. 2.2 Safety Precautions in the Microbiology Laboratory in Ekiti State University, Ado-Ekiti People must exercise care and common sense when working with microorganisms. Bench work tops and incubators should be disinfected regularly. An operation that might generate infection. Aerosols should be carried out in a biological safety cabinet. Autoclave must be maintained and operated properly to ensure adequate sterilization. Laboratory personnel should wash their hands thoroughly before and after work. Dispose of all waste safely. Do not overfill discard containers. Use appropriate disinfectant. Use separate container for sharps. Avoid spillage by using racks to hold containers. Work neatly and keep the bench surface free of any unnecessary materials. Wear protected gloves, Wearing of face mask for all procedure involving direct contact with infections materials. When wearing gloves, the hands should be washed with the gloves on particularly before using the telephone or doing clerical works. 4 Always wash your hands after handling infectious materials. When leaving the laboratory and before attending patients. 2.2 Cover open wounds with water proof dressing. Wearing of laboratory coats is must and necessary. Equipment and Machine Used in the Laboratory Equipment being used in the department of microbiology laboratory of Ekiti State University, Ado-Ekiti includes: Fig. 2.1: Microscope Microscope: this is the instrument used in the laboratory for viewing tiny microorganisms that cannot be seen with your naked eye. The microscope employs a hollow, extremely intense cone of light concentrated on the specimen. The field of view of the objective lens lies in the 5 hollow, dark portion of the cone and picks up only scattered light from the object. The clear portions of the specimen appear as a dark background, and the minute objects under study glow brightly against the dark field. This form of illumination is useful for transparent, unstained biological material and for minute objects that cannot be seen in normal illumination under the microscope Fig. 2.2: Incubator Incubator: this is an instrument in which organisms are allowed to grow in cultures because of the favorable temperate. The incubator can be set to normal temperature for the growth of organisms (35°C - 37°C). The cultures are left inside the incubator for 18 – 24 hours. 6 Fig 2.4: Autoclave Autoclave: The autoclave is effective equipment used for steam sterilization at pressures above the atmospheric pressure. Thus, it is possible to steam at higher temperature then the boiling point, which a lot of microorganisms cannot withstand. Autoclaving is the most effective method for sterilizing culture media. When sterilizing culture media with autoclave, we do so at 1.05Kg per square centimeter for 15 minutes to eliminate contaminations. Procedure using an Autoclave First, water inside the autoclave must be up to normal level. The Medias to be sterilized are loaded inside the try, then we put the upper try and load the bottle (to be used in pouring the Medias after sterilization) inside the top try, it was covered with the lid and the upper lid is fastened down and the valves closed, the temperature is set to 121°C for 15 minutes. It is then switched on and the water starts to boil, when pressure rises to the normal range, the valve is closed and the temperature start rising, when it reaches 121°C, the autoclave is switched off and the pressure is allowed to cool down until it reaches zero. 7 The value is opened and the remaining pressure is allowed to come out after which the lid is unfastened and the Medias are taken out for pouring. Fig 2.5: Weighing Balance Weighing balance: an instrument used for weighing media and any order reagent depending on the limit of the weight of the balance. 8 Fig. 2.6: Refrigerator Refrigerator:. This is used to preserve samples, reagents etc, which are used for daily analysis and cannot be exhausted at once. The refrigerator helps provide optimum environment for materials to be preserved 9 Fig 2.7: Centrifuge Centrifuge: it is used for spinning liquid/fluid sample e.g. urine or blood etc. At different revolution for specific minute e.g. i. Spinning of blood samples to separate the red blood cell from the plasma. ii. Spinning of urine sample to separate the supernatant from the deposit. Water Bath: This is required to incubate bottle of culture media, liquids in flasks or other large Containers, and when incubating samples in the test tube racks. Straight Wire: It is made up of a thick metallic lower part and a straight thin upper metallic part usually made up of platinum. This straight wire is used for stab culture and for picking discrete colonies. Usually sterilized before, during and after usage. This is achieved by flaming on Bunsen burner red hot and allowed to cool a bit before use. Wire Loop: Made up of a thick metallic lower part and a straight thin upper metallic part curved into a small circle usually made up of platinum. Wire loop is used generally for inoculating samples and picking colonies sterilized by flaming red hot before, during and after use. It is always better to use the sides of the loop rather than the apex during inoculation. 10 Mycology Needle: It is made up of a thick metallic lower part and a short straight thin upper metallic part usually made up of platinum. Used for needle mount preparations of fungi and fungi inoculation. It is usually sterilized by flaming. ` Glass Slides: Used for preparation of slides for microscopy. Sterilization is by flooding with alcohol and flaming off excess alcohol. Cover Slips This is use for covering wet smears of preparations. It is sterilized by flooding with alcohol and flaming off excess alcohol. Petri Dish: Used for the preparation of culture media. It is usually bought sterilized. The disposable type cannot be used a second time while the glass ware type can be reused be usually sterilized by autoclaving. Forceps: A pair of forceps is a metallic object used for handing hot object or contaminated materials. It is sterilized by flaming red hot. Other Laboratory equipment include includes sterilized slide, Giemsa Stain, needle, syringe, ethanol, sterilized bottle, agar (MacConkey or Chocolate), Gram positive, Gram negative sensitivity kit , cotton wool, EDTA, microscope, ethanol, sterilized slides, swab sticks, cotton wool, spirit, giemsa stain, lancets, surgical blades, oil immersion, pipette, light microscope, hot plate (dryer), hand gloves, capillary tubes for measuring PCV, sealant, microhaematocrit reader, anaerobic jar, test tubes, bottles, water bath, weighing balance, microscope, pipette, beakers, bio safety cabinet, cotton. 11 2.3 Organogram Vice Chancellor Dean, Faculty of Science Head of Department, Microbiology Chief Laboratory Technologist Laboratory Technologist Postgraduate Students Laboratory Technician Postgraduate Students Store Keeper 12 Laboratory Attendants Cleaning Staff 2.4 Good Microbiological Laboratory Practice (GMLP) Working safely in the laboratory and producing quality research are key principles of any research program. Good microbiological laboratory practices (GMLP) are designed to protect both workers (i.e., lab staff, non-lab staff and students) and research material (i.e., organisms and equipment). Laboratory biosafety is primarily achieved through a basic level of operational practices that include good microbiological laboratory practices and physical design features of a functional well-designed laboratory. Good microbiological laboratory practice includes the following: 1. A documented procedural (safety) manual must be available for all staff and students; 2. Personnel must receive training on the potential hazards associated with the work involved and the necessary precautions to prevent exposure to biological agents and release of contained material; 3. Eating, drinking, smoking, storing of either food, personal belongings, or utensils, applying cosmetics, and inserting or removing contact lenses are not permitted in any laboratory; 4. Open wounds, cuts, scratches and grazes should be covered with waterproof dressings; 5. Oral pipetting of any substance is prohibited in any laboratory; 6. Long hair is to be tied back or restrained so that it cannot come into contact with hands, specimens, containers or equipment; 7. Access to laboratory and support areas is limited to authorized personnel; 8. Open wounds, cuts, scratches and grazes should be covered with waterproof dressings; 9. Laboratories are to be kept clean and tidy. Storage of materials that are not pertinent to the work and cannot be easily decontaminated (e.g., journals, books, correspondence) should be minimized; 10. Laboratory coat, properly fastened and suitable footwear must be worn by all personnel; 11. If a known or suspected exposure occurs, contaminated clothing must be decontaminated before laundering (unless laundering facilities are within the containment laboratory and have been proven to be effective in decontamination); 12. Wear approved safety glasses and/or goggles; 13. Hands must be washed after gloves have been removed, before leaving the laboratory and at any time after handling materials known or suspected to be contaminated; 13 14. Gloves (e.g., latex, nitrile) must be worn for all procedures that might involve direct skin contact with biological material; 15. The use of needles, syringes and other sharp objects should be strictly limit. It is recommended to use safety-engineered medical sharps whenever possible; 16. Use disinfectant traps and in-line filters on vacuum lines to protect vacuum lines from potential contamination; 17. Work surfaces must be cleaned and decontaminated in accordance with biological material in use at the end of the day and after any spill of potentially biohazardous materials; 18. Contaminated materials and equipment leaving the laboratory for servicing or disposal must be appropriately decontaminated and labelled or tagged out as such; 19. Autoclaves used for decontamination need to have regular efficacy monitoring with biological indicators; 20. All biological materials, solid or liquid, must be decontaminated before disposal or reuse; 21. Disinfectants effective against the agents in use must be available at all times within the areas where the biological material is handled or stored; 22. Leak-proof containers are to be used for the transport of biological materials; 23. Spills, accidents or exposures to biohazardous materials and losses of containment must be reported immediately to the laboratory technologists; 24. An effective rodent and insect control program must be maintained. 14 CHAPTER THREE VARIOUS BIOCHEMICAL TESTS PERFORMED IN LABORATORY Various activities and test were carried out in the microbiology laboratory, these include adhering to good microbiological laboratory practice (GMLP), the use of microbiology laboratory apparatus, how to prepare nutrient agar, how to sterilize nutrient agar, serial dilution, spill management, culture plating techniques and isolation of culture, gram staining, biochemical tests, sugar fermentation etc. 3.1 Preparation of Nutrient Agar Nutrient agar contains nutrients that suitable to subculture a wide range of microorganisms and makes it an excellent agar media to check on the purity before any biochemical or serological test. Materials Required: Nutrient agar powder/dehydrated medium Measuring cylinder Weighing balance Conical flask Cotton wool Aluminum foil Autoclave tape Autoclave Sterile petri dishes pH meter Distilled water Bunsen burner Marker pen Laboratory coat and gloves Procedure: 1. Preparation of Equipment: o Clean all glassware thoroughly o Dry the glassware properly o Wear appropriate personal protective equipment 2. Medium Calculation and Weighing: o Calculate required amount (typically 28g per liter) 15 o Weigh the nutrient agar powder using analytical balance o Record the weight accurately 3. Suspension of Medium: o Measure appropriate volume of distilled water in measuring cylinder o Transfer water to conical flask o Add weighed nutrient agar powder gradually while stirring o Mix thoroughly to avoid clumping 4. Heating Process: o Heat the mixture with frequent agitation o Continue heating until powder completely dissolves o Solution should become clear and homogeneous 5. pH Adjustment: o Allow solution to cool slightly o Check pH using pH meter o Adjust to 7.4 ± 0.2 at 25°C if necessary o Using NaOH or HCl for adjustment 6. Flask Preparation for Sterilization: o Plug flask with cotton wool o Cover with aluminum foil o Label flask with contents and date o Apply autoclave tape 7. Sterilization Process: o Place in autoclave o Sterilize at 121°C, 15 psi for 15-20 minutes o Allow autoclave to cool naturally o Remove medium when pressure reaches zero 8. Pouring Plates: o Cool medium to 45-50°C (hand-touch temperature) o Flame the neck of the flask o Pour approximately 20-25ml into each sterile petri dish o Pour near Bunsen burner flame 16 o Avoid bubbles during pouring 9. Plate Setting: o Allow plates to solidify at room temperature o Keep plates slightly open to prevent condensation o Once solidified, close and invert plates 10. Quality Control: o Check for: Proper solidification Absence of bubbles Uniform thickness No contamination o Incubate one plate for sterility check 11. Storage: o Stack plates invertedly o Store at 4°C in sealed plastic bags o Label with preparation date o Use within specified time period This medium serves as a basic growth medium for non-fastidious microorganisms and is fundamental in microbiological work. Proper preparation ensures reliable results in subsequent microbiological procedures. 3.2 Serial Dilution 3.3 Culture plating techniques and isolation of culture 3.5 Gram staining This is used to differentiate the morphology (shape and size) and to demonstrate a method of differentiating bacterial into groups, namely; the gram positive (those retaining the blue color) and the gram negative (those which can be decolorized and counterstained in red). There are two types of gram staining procedure, there are direct and indirect gram staining procedures. 17 Direct Gram Staining Procedure; Rob the swab stick containing the sample on a clean grease-free slide. Air-dry and heat-fix. Stain the slide with grams crystal violet solution (primary dye) and leave for 30 seconds. Flood the slide with water and drain off. Add lugol’s iodine solution to the slide and wash after 30 seconds. Place the slide slantly and decolorized with alcohol or acetone drop wise, until the blue color has been removed. Wash off with water drain off the side. Flood the safranin (counterstained) for 30 seconds. Wash and air –dry. Examine the smear under the oil immersion objective lens (x 100). Indirect Gram Staining Procedure; Put a loop full of normal saline on a slide with sterile wire loop. Sterile standard wire loop was then used to pick a colony of the organism and smeared together with normal saline. Air-dry and then heat fix. Flood the slide with crystal violet solution for 30 seconds Wash the slide with water and drain off water Add lugol’s iodine and wash after 30 seconds Decolorize the slide slantly with acetone or alcohol, until the blue color removed. Wash with water and drain Examine the smear under the oil immersion objective lens (x 100) RESULT: Gram positive organisms appear dark purple eg staphylococci, streptococci, micrococci, pneumococci, enterococci, diphtheria bacillus. Gram negative organisms appear light red or pink. eg. gonococci, meningococci, coliform bacilli, shigella, salmonella, vibrio. Tissue cells, leucocytes and debris in inflammatory exudates all stain gram negatively. 3.6 Biochemical tests 18