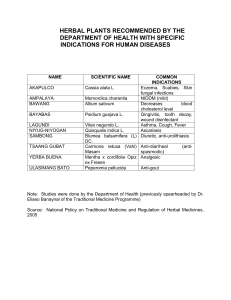
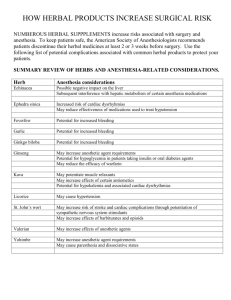
APPLIED AND FORENSIC PHARMACOGNOSY (PG 706) Prof. Dina Abou-Hussein; Email: dina.abouhussein@pharma.cu.edu.eg Protection or safeguard of a public health can be established by ensuring appropriate standards of safety, quality and efficacy for medicines available on the market. The safety and efficacy of herbal medicines largely depend on their quality. Learning Outcomes ➢ Define Quality management in Drug Industry and determine its components. ➢ Highlight the importance of Quality Assurance in the manufacture of herbal medicines. ➢ Recognize Good Manufacturing practice for herbal medicines. ➢ Understand the challenge in applying Quality control in herbal drugs. ➢ Identify the Problems facing QC analysis of herbal drugs. Quality management (QM) in the Drug Industry • QM: is defined as the aspect of management function that determines and implements the “quality policy”. • The concepts of Quality Assurance (QA), Good Manufacturing Practice (GMP) and Quality Control (QC) are interrelated aspects of quality management. QA GMP QC QA GMP QC Quality Management for Herbal Medicines 1- Quality Assurance in the manufacture of herbal medicines: • It is the totality of the arrangements made with the purpose of ensuring that pharmaceutical products are of the quality required for their intended use. • The system of quality assurance appropriate to the manufacture of herbal medicine should ensure that: a) Requirements of GMP and other associated codes such as of Good Laboratory Practice (GLP) and Good Clinical Practice (GCP) are adopted. b) Production and control operations are clearly specified in a written form. c) Managerial responsibilities are clearly specified in job descriptions. d) All necessary controls on starting materials, intermediate products and bulk products and other in-process controls, calibrations and validations are carried out. 2- Good manufacturing practice for herbal medicines: • GMP is that part of quality assurance which ensures that products are consistently produced and controlled to the quality standards appropriate to their intended use and as required by the marketing authorization. • In addition, there are supplementary guidelines specifically in relation to the production and control of herbal medicines. Collection/cultivation of medicinal plants should follow relevant guidance such as the WHO guidelines on Good Agriculture and Collection Practices (GACP) for medicinal plants. 2- Good manufacturing practice for herbal medicines: • Under GMP: a) All manufacturing practices are clearly defined, systematically reviewed and comply with specifications of the required quality of the pharmaceutical products. b) Qualification and validation are performed: The reproducibility of the production process is the main means for ensuring consistency of quality, efficacy and safety between batches. c) All necessary resources are provided. d) Instructions and procedures are written in clear language. e) Operators are trained to carry out procedures correctly. f) Records are made during manufacture to show that the quality and quantity of the product are as expected; any significant deviations are investigated. g) Records covering manufacture and distribution enable the complete history of a batch to be traced. 2- Good manufacturing practice for herbal medicines: • Under GMP: h) A high level of sanitation and hygiene during manufacture is necessary. i) A proper storage of products minimizes any risk to their quality. j) Complaints about marketed products are examined, the causes of quality defects investigated, and appropriate measures taken in respect of the defective products to prevent recurrence. They are basically two types of complaints 1) product quality complaints 2) adverse reactions/events. k) A system is available to recall any batch of product from sale or supply. There should be a Standard Operating Procedure (SOP) for storage of recalled herbal medicines in a secure segregated area. l) Personnel: the release of herbal medicines should be authorized by a person who has been trained in the specific features of the processing and quality control of herbal materials, preparations and products. Training records should be maintained and periodic assessments of the effectiveness of training programmes should be made. 2- Good manufacturing practice for herbal medicines: • Under GMP: m) Premises should be designed, located, constructed, adapted and maintained to suit the operations to be carried out according to WHO good manufacturing practices for pharmaceutical products. n) Reference samples and standards should be of a quality appropriate to their purpose. They should be stored under appropriate conditions to prevent degradation. Their expiry and/or revalidation date should be determined and indicated. o) Effective cleaning of the equipment is particularly important specially because of the processing of herbal materials that may generate dust or material which is susceptible to pest-infestation or microbiological contamination and cross contamination. 2- Good manufacturing practice for herbal medicines: • Under GMP: p) Documentation: Essential part of the QA and should exist for all aspects of GMP To ensure that: 1. The specifications and procedures for all materials and methods of manufacture and control are defined. 2. All personnel concerned with manufacture know what to do and when to do it. 3. Authorized persons have all the information necessary to decide whether or not to release a batch of a drug for sale. 4. Documented evidence, records and audit track that will permit investigation exists. 5. The data needed for validation; reviews of statistical analysis are available. 3- Quality Control of Herbal Drugs Introduction Is it worthy to apply QC on herbal medicinal products? • Herbal medicinal products play an important role not only in self-medication but also in medical practice. • Many physicians believe that herbal medicinal products are able to beneficially complement or even replace chemical medicines and that they have less or even no side effects. • There is also a preference consumers for natural therapies. • Manufacturers should accept the challenge to fulfill the expectations related to quality, safety and efficacy of these products. • Herbal medicinal products are always mixtures of a number of substances. • Quality control is applied on starting raw materials, as well as on finished products. • The quality of finished products is influenced by the quality of the raw materials used. Quality control is applied in two stages. A) QC of starting raw material used in manufacture: e.g. Dried mentha leaves, mentha oil, mentha extract To enusre identity (authentic), pure (not cantaminated or adulterated), content (yield of oil, % of menthol). B) QC of finished product: e.g. Mentha herbal tea, products containing oil (capsule, cream, syrup, etc.) products containing mentha extract (capsule, syrup, etc.) Mentha extract Mentha dry leaves Mentha oil Learning Outcomes ➢ Compare between the specifications of herbal materials and those of herbal finished products. ➢ Define herbal drugs, herbal preparations and herbal extracts. ➢ Classify and Differentiate between different types of herbal extracts . ➢ Identify the Problems facing QC analysis of herbal drugs. What are the specifications of Herbal materials? The specifications of finished herbal products? Specifications of Herbal Materials: • The family and botanical name of the plant used according to the binomial system. • Details of the source of the plant. • Details of which part of the plant is used. • For dried materials, the drying system should be specified. • A description of the plant material based on macroscopic and microscopic examination. • Suitable identity tests. • Details of the assay. • Limit tests such as dry residue of liquids. • Suitable methods for the determination of possible pesticide, toxic material and microbial contamination. Specifications of Finished Herbal Products: • Tests for microbiological contamination and tests for other toxicants. • Uniformity of weight, disintegration time, hardness and friability, viscosity, consistency and dissolution, if applicable. • Physical appearance such as color, odor, form , shape, size and texture. • Loss on drying, or water content. • Identity tests, qualitative determination of relevant substances of the plants (e.g. fingerprint chromatogram). • Quantification of relevant active ingredients, if they have been identified, and the analytical methods that are available. • Limit tests for residual solvents. Finished herbal products may consist of herbal preparations made from one or more herbs. They may also contain excipients in addition to the active ingredients. Define: •Herbal drugs •Herbal drug preparations •Herbal extracts (classify herbal extracts) Definitions: • Herbal drugs: are mainly whole, fragmented or cut, plants, parts of plants, algae, fungi, lichen in an unprocessed state, usually in a dried form, but sometimes fresh. Certain exudates that have not subjected to a specific treatment are also considered to be herbal drugs. • Herbal drug preparations: are obtained by subjecting herbal drugs to treatments such as extraction, distillation, expression, fractionation, purification, concentration and fermentation. These include comminuted or powdered herbal drugs, tinctures, extracts, essential oils, fatty acids, expressed juices and processed exudates. • Herbal extracts: are preparations of liquid, semi-solid or solid consistency, obtained from herbal drugs. Herbal extracts are classified I- in terms of their physical state: liquid, semi-solid (soft) and dry extracts. II- in terms of containing 1- only herbal extractable matter (never contain excipients or residual solvents)= Native extracts. 2- additional excipients (technical excipients needed for adjustment or extraction solvent)= Not-native extracts. III- in terms of knowledge relating to therapeutically active constituents: 1- Adjusted/ standardized extracts 2- Quantified extracts 3- Other extracts. Definitions: • Standardized extracts: are adjusted within a narrow tolerance to a given content of constituents with known therapeutic activity. (Adjustment is carried out with inert excipients or by blending of production batches with a higher or lower content of therapeutically active constituents). E.g. Belladonna leaf dry extract, tincture 0.95% - 1.05% of total alkaloids, expressed as hyoscyamine. • Quantified extracts: are adjusted to a defined range of constituents which are accepted to contribute clinical or pharmacological efficacy. (Adjustment can only be achieved by blending of batches of extracts with the same specifications). E.g. Ginkgo dry extract (22-27% flavonoids, 2.6-3.2% bilobalide, 2.8-3.4% ginkgolides). Marker substances are chemically defined constituents of a herbal drug that are important for the quality of the finished product whether it is responsible for pharmacological activity or not. Definitions: • Other extracts: are extracts in which constituents with therapeutic activity or active markers are unknown. They are characterized by a constant amount of native herbal drug preparation and of inert excipients. Problems facing QC analysis of herbal drugs (phytomedicines): Related to the biological variability ▪ Chemo-varieties and chemo cultivars exist. ▪ The source and quality of the raw material are variable. ▪ The methods of harvesting, drying, storage, transportation, and processing have an effect. Related to the marker selection: ▪ Herbal drugs are usually mixtures of many constituents. ▪ The active principle(s) is (are), in most cases unknown. ▪ Selective analytical methods or reference compounds may not be available commercially. ▪ Sometimes chemical markers used for QC purposes, have no therapeutic activity. Therefore strict guidelines have to be followed for the successful production of a quality herbal drug. Among them are : ✓ proper botanical identification ✓ phytochemical screening ✓ standardization Standardization of Herbal Drugs Standardization is the basic prerequisite for consistent efficacy of herbal products. It is the body of information and controls that are necessary to guarantee the constancy of composition, and consequently the constancy of activity, of an herbal medicine. Is it easy to obtain standardized herbal medicinal products? Standardization is a hard task and involves: 1- Standards relating to the herbal drug: - Cultivating the plant according to Good Agricultural Practice (GAP). - Setting a detailed specification/monograph of the herbal drug. 2- Standards related to the herbal drug preparations: - The extraction solvent (type and concentration). - Manufacturing process. - chemical isolation and characterization of active reference substances. - Setting up validated analytical methods. - In-process-control. 3- Standards related to the finished herbal products: - Good Manufacturing Practice (GMP). - Setting detailed specifications of the final product. Marker Substances: • What does it mean? • What is the purpose of its presence in an herbal preparation? • How to select the marker? • Should the marker be stated in the label? Learning Outcomes ➢ Define Marker substance and criteria of its selection. ➢ Differentiate the categories of marker substances. ➢ Highlight the importance of marker substances. ➢ Use of Fingerprint chromatograms producing full spectrum standardized herbal extracts for authentication, identification and quality control purposes of herbal products. ➢ Distinguish plants by DNA fingerprinting. Marker Substances: ????????????????? • What does it mean? • What is the purpose of its presence in an herbal preparation? • How to select the marker? • Should the marker be stated in the label? 30 Marker Substances: Definition: Marker substances or groups of marker substances are chemically defined constituents of herbal drugs, herbal drug preparations and herbal medicinal products which, according to the state of scientific knowledge, do not contribute to therapeutic activity. They only serve analytical purposes. 31 Marker Substances: Categories of Markers: 1. Markers which are characteristic for the respective genus or family of the plant (suitable for identification tests and assay). 2. Markers which occur universally in plants (quantification). 32 Marker Substances: Importance of the Marker: 1. Identification testing . 2. Evidence for the consistency of the manufacturing process (batch – to batch control). 3. Stability testing (batch – to batch conformity over the whole shelf-life). 33 Marker Substances: Criteria of Marker Selection: A constituent of an herbal drug is eligible as a marker 1. If it is specific for the respective test 2. If selective determination among all other constituents is possible. 3. Other criteria : stability and availability 34 Marker Substances: N.B.: As marker substances serve only analytical purposes: ▪ Adjustment to a defined content of these substances is not allowed. ▪ The content of marker substances must not be stated in the label . Because this would evoke expectations regarding the therapeutic quality of the product which have no scientific background. 35 There is often disagreement among researchers as to which compound is considered to be the “active” one. 36 A compound that may be “inert” today, may be considered “active” tomorrow 37 It does not suffice to identify the respective zone in the chromatogram of the test solution 38 Explain how herbal fingerprints can be developed by a multitude of chemical analytical methods. Full Spectrum Standardized Herbal Extracts The most current approach in herbal validation and standardization is herbal analysis of the whole herb instead of its component parts. As far as the standardization of extracts is concerned, the aim has to be the reproducibility of all the chemical components contained in an extract, including the unknown ones. Fingerprint chromatograms must conform to the total spectrum of constituents. 40 Full Spectrum Standardized Herbal Extracts I- Chromatographic Fingerprint ▪ Thin Layer Chromatography ▪ Gas Chromatography ▪ High Performance Liquid Chromatography ▪ Hyphenated Procedures II- DNA Fingerprint 41 Full Spectrum Standardized Herbal Extracts I- Chromatographic Fingerprint ▪ Strongly recommended for the purpose of quality control of herbal medicines. ▪ They represent appropriately the “chemical integrities” of the herbal medicines. ▪ Used for authentication and identification of the herbal products. 42 Full Spectrum Standardized Herbal Extracts I- Chromatographic Fingerprint ▪ A chromatographic pattern of pharmacologically active and or chemically characteristic constituents present in the extract. ▪ Demonstrates both “sameness” “differences” between various samples. and ▪ Evaluates the quality of herbal medicines globally considering multiple constituents present in the herbal medicines. 43 Chromatographic Fingerprint: High Performance Liquid Chromatography (HPLC) HPLC Fingerprint chromatogram of the spectrum of constituents of five batches of Hypericum extract (extraction solvent: ethanol 60%) – conformity of batches 44 HPTLC Analysis of Valerian Species 1: Valeriana officinalis ( authenticated ) 2: Valeriana officinalis (Pacific source) 3: Valeriana officinalis (Dutch source) 4: Valeriana radix (PHH8) 5: Valerenic acid 6: Valeriana stichensis 7: Valeriana wallichi (Indian Valerian) HPLC Chromatographic fingerprint of different DGL extracts (DGL-Ev, DGL-Ge & DGL-Ph) 140 UV DGL (EVA) 051DGL (EVA).dat UV DGL (Gemini) 049DGL (Gemini).dat 140 UV DGL (phyto) 050DGL (phyto).dat 120 120 Liquiritigenin 100 80 80 60 60 40 40 DGL-Ev 20 20 DGL-Ge DGL-Ph 0 0.0 2.5 5.0 7.5 10.0 12.5 15.0 Minutes 17.5 20.0 22.5 0 25.0 27.5 30.0 mAU mAU 100 Fingerprints of EtOAc & But. Extract of Passiflora Stability Study by Fingerprint Chromatogram Chromatographic Fingerprint: Hyphenated Procedures Combining ▪ A chromatographic separation system with ▪ A spectroscopic separation system with ▪ A spectroscopic detector In order to obtain structural information on the analytes present in a sample 49 Chromatographic Fingerprint: Difficulties in Development of Chromatographic Fingerprints for Herbal Medicines: 1. When herbal drugs are considered for analysis, a large number of chemical components are involved and many of them are in low concentration 2. Chromatographic instruments and experimental conditions are difficult to reproduce during real analysis. Therefore several data treatments would be needed during fingerprint analysis. 50 Chromatographic Fingerprint: Chemometric Approaches and Data Processing for Chromatographic Fingerprints of Herbal Medicines: Chemometrics is the science of extracting information from chemical systems by datadriven means. This can be used to compare common pattern of the chromatographic fingerprints obtained. Due to complexity of the chromatographic fingerprint and the irreproducibility of chromatographic instruments and experimental conditions 51 DNA Fingerprinting: DNA Fingerprinting is being applied in: 1. Authentication of medical herbs 2. Quality Control of medical herbs 3. Protection of property rights for new plant 52 DNA Fingerprinting: ▪ DNA fingerprint is generally independent of environment ▪ It is consistent throughout different parts and developmental stages 53 DNA Fingerprinting: ▪ Similarity of DNA fingerprints depends on genetic closeness of tested samples ▪ DNA fingerprinting can distinguish plants from different families, genera, species, or cultivars (cultivated variety) ▪ Clones have the same DNA fingerprint as their mother plant 54 55 DNA Fingerprinting: 56 Combining the use of DNA Fingerprinting & Chemical Fingerprinting will be an effective tool in Authentication and Quality Control of Herbs 57 Stability By means of appropriate fingerprint chromatogram, it must be shown that all ingredients in the preparation are stable and that their proportional content remains constant. Example of a Certificate of Analysis ▪ ▪ Name of the product: Content of the product: ▪ Batch number: ▪ Manufacturing date: ▪ Expiry date: 1. Organoleptic properties: Color: Odor: Taste: 2. Determination of certain Pharmacopoeial constants: 1. Determination of extractable matter 3. Macroscopic Investigation: 4. Microscopic Examination: 5. Chromatographic analysis: TLC (2D fingerprint) No. of the spots-Rf values-Intensity of the spots HPLC analysis No. of peaks-Rt of peaks-Area of peaks-Concentration of the markers GC analysis of volatile compounds 2. Determination of water and volatile matter No. of peaks-Rt of peaks-Area of peaks-Concentration of the markers 3. Determination of volatile oils 6. Use of NMR Spectroscopy and Multivariate Analysis. 4. Determination of bitterness value 7. Test for pesticide residue. 5. Determination of haemolytic activity 8. Determination of arsenic and heavy metals. 6. Determination of tannins 9. Determination of microorganisms. 7. Determination of swelling index 8. Determination of foaming index 10. Radioactive contamination. References: Pharmacognostic Methods for Analysis of Herbal Drugs, According to European Pharmacopoeia: www.intechopen.com Identification, evaluation and standardization of hebal drugs: A review: Der Pharmacia Lettre, 2010, 2(6): 302-315 Facts about standardization of herbal medicine: a review: Journal of Chinese Intergrative Medicine, October 2012, Vol.10, No. 10. Quality Control of Herbal Medicines: From Traditional Techniques to State-ofthe-art Approaches: Planta Med 2021; 87: 964–988 References: Egyptian Guidelines For Registration of Herbal Medicines: Arab Republic of Egypt, Egyptian drug authority (EDA), Central Administration for Pharmaceutical products (2021) Egyptian Herbal Monograph: Egyptian Drug Authority (EDA) 2022 Guideline on quality of herbal medicinal products/traditional herbal medicinal products: European Medicines Agency, 2022