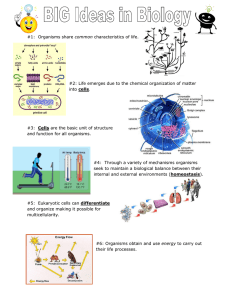
1 | The Foundations of Biochemistry © 2017 W. H. Freeman and Company CHAPTER 1 The Foundations of Biochemistry Learning goals: • Distinguishing features of living organisms • Structure and function of the parts of the cell • Roles of small and large biomolecules • Energy transformation in living organisms • Regulation of metabolism and catalysis • Coding of genetic information in DNA • Role of mutations and selection in evolution Biochemistry Is the Chemistry of Living Matter Living matter is characterized by: • a high degree of complexity and organization • the extraction, transformation, and systematic use of energy to create and maintain structures and to do work • the interactions of individual components being dynamic and coordinated • the ability to sense and respond to changes in surroundings • a capacity for fairly precise self-replication while allowing enough change for evolution Complexity and Organization Living Organisms Must Intake and Transform Nutrients into Energy Living Organisms Must Accurately Reproduce Three Distinct Domains of Life Defined by Cellular and Molecular Differences That Evolved over Time Six Kingdoms of Life Defined by Organism, Cellular, and Molecular Differences Six kingdoms Cellular organization • Archaea • Bacteria • Protista • Fungi • Plantae • Animalia Unicellular prokaryote Unicellular prokaryote Unicellular eukaryote Uni- or Multicellular eukaryote Multicellular eukaryote Multicellular eukaryote Cell: The Universal Building Block • Living organisms are made of cells. • The simplest living organisms are unicellular (singlecelled). • Larger organisms are multicellular (many-celled), with different functions for different cells. • Cells have some common features but can contain unique components for different organisms. All Cells Share Some Common Features Bacterial Cell Structure Components of Bacterial Cell Structure Composition Function Cell wall Cell membrane Nucleoid Ribosomes Pili Flagella Cytoplasm Carbohydrate + protein Lipid + protein DNA + protein RNA + protein Protein Protein Aqueous solution Mechanical support Permeability barrier Genetic information Protein synthesis Adhesion, conjugation Motility Site of metabolism Eukaryote Cells: More Complexity • Have membrane-bound nucleus by definition: – protection for DNA; site of DNA metabolism – selective import and export via nuclear membrane pores • Have membrane-enclosed organelles: – mitochondria for energy in animals, plants, and fungi – chloroplasts for energy in plant – lysosomes for digestion of un-needed molecules • Compartmental segregation of energy-yielding and energy-consuming reactions helps cells to maintain homeostasis and stay away from equilibrium. Animal and Plant Cells Contain Unique Components Animal and Plant Cells Contain Unique Components Animal and Plant Cells Contain Identical and Unique Components Plant Both Animal Chloroplast Vacuole Glyoxysome Plasmodesma Cell wall Membrane Nucleus and Nucleolus Mitochondria Rough and Smooth ER Ribosomes Golgi Cytoskeleton Lysosome Peroxisomes Cytoplasm and Cytoskeleton • Cytoplasm is a highly viscous solution where many reactions take place. • Cytoskeleton consists of microtubules, actin filaments, and intermediate filaments. – cellular shape and division – intracellular organization – intracellular transport paths – cellular mobility The Cytosol Is Very Crowded Folded proteins Translated peptide Cytoskeleton Maintains Cellular Organization Cellular Organization Is Dynamic, Changing Drastically at Different Stages Biochemistry is the Chemistry of Living Matter • The basis of all life is the chemical reactions that take place within the cell. Chemistry allows for: • a high degree of complexity and organization • the extraction, transformation, and systematic use of energy to create and maintain structures and to do work • the interactions of individual components to be dynamic and coordinated • the ability to sense and respond to changes in surrounding • a capacity for fairly precise self-replication while allowing enough change for evolution Organisms Can Also Be Classified by Different Energy and Carbon Sources Living Systems Extract Energy • From sunlight – plants – green bacteria – cyanobacteria • From fuels – animals – most bacteria • Energy input is needed in order to maintain life. The Molecular Logic of Life We look at the chemistry that is behind the: • initiation and acceleration of reactions • organization and specificity of metabolism and signaling • storage and transfer of information and energy The Molecular Hierarchy of Structure Biochemistry: Unique Role of Carbon 30 Elements Essential for Life • Other than carbon, elements H, O, N, P, and S are also common. • Metal ions (e.g., K+, Na+, Ca++, Mg++, Zn++, Fe++) play important roles in metabolism. Common Functional Groups of Biological Molecules Biological Molecules Typically Have Several Functional Groups The ABCs of Life The Function of Molecules Strongly Depends on Three-Dimensional Structure Cis vs. Trans Cis vs. Trans Enantiomers and Diastereomers Diastereomers (non-mirror images) Enantiomers and Diastereomers Interactions Between Biomolecules Are Specific • Macromolecules fold into 3D structures with unique binding pockets. • Only certain molecules fit in well and can bind. • Binding of chiral biomolecules is stereospecific. Interactions Between Biomolecules Are Specific Organisms Perform Energy Transductions to Accomplish Work to Stay Alive Organisms Perform Energy Transductions to Accomplish Work to Stay Alive How to Speed Reactions Up Higher temperatures − stability of macromolecules is limiting Higher concentration of reactants − costly, as more valuable starting material is needed Changing the reaction by coupling to a fast one − universally used by living organisms Lower activation barrier by catalysis − universally used by living organisms Equilibrium and ΔG°Measure Spontaneity of a Reaction Unfavorable and Favorable Reactions Energy Coupling • Chemical coupling of exergonic and endergonic reactions allows otherwise unfavorable reactions. • The “high-energy” molecule (ATP) reacts directly with the metabolite that needs “activation.” Catalysis • A catalyst is a compound that increases the rate of a chemical reaction. ‡ • Catalysts lower the activation free energy G . • Catalysts do not alter G°. • Enzymatic catalysis offers: – acceleration under mild conditions – high specificity – possibility for regulation Enzymes Lower the Activation Energy to Increase the Reaction Rate Series of Related Enzymatically Catalyzed Reactions Forms a Pathway Metabolic pathway • produces energy or valuable materials Signal transduction pathway • transmits information Pathways Are Controlled in Order to Regulate Levels of Metabolites Example of a negative regulation: Product of enzyme 5 inhibits enzyme 1 to prevent wasteful excess products. Genetic and Evolutionary Foundations • Life on Earth arose 3.5–3.8 billion years ago. • The formation of self-replicating molecules was a key step. • Could it have been DNA? • Could it have been proteins? RNA World? • RNA can act both as the information carrier and biocatalyst. • Some viruses use RNA as a primary means of genetic information. Complementarity in DNA Allows for Replication with Near-Perfect Fidelity The Central “Dogma” of Biochemistry: DNA → RNA → Protein Natural Selection Favors Some Mutations • Mutations occur more or less randomly in DNA and RNA. • Mutated polynucleotides may be transcribed and translated into molecular machinery like proteins. • Mutations that give organisms an advantage in a given environment are more likely to be propagated. Natural Selection Favors Some Mutations Evolution of Eukaryotes Could Also Be Mediated Through Endosymbiosis Chapter 1: Summary In this chapter, we learned to: • understand what defines living organisms • relate structure and function of the cell • realize that the structure of biomolecules often gives them specific functions • grasp principles of bioenergetics • review the forces behind evolution