E BOOK 1st Edition Hox Modules in Evolution and Development David E. K. Ferrier
advertisement

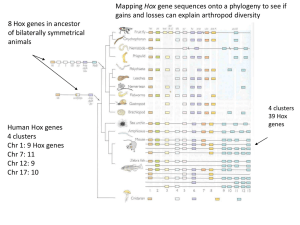
Get all Chapters For Ebook Instant Download by email at etutorsource@gmail.com Contents . . Chapter 1 Introduction to Hox Modules in Evolution and Development..........................................................................................1 Chapter 2 Multiple Layers of Complexity in the Regulation of the Bithorax Complex of Drosophila....................................................... . 15 Chapter 3 The Role of Hox Genes in the Origins and Diversification of Beetle Horns........................................................................................ 53 Chapter 4 Duplication and Evolution of Hox Clusters in Chelicerata (Arthropoda)........................................................................................ 77 Chapter 5 Structural Constraints in Hox Clusters: Lessons from Sharks and Rays............................................................................................ 103 Chapter 6 . Evolution of Cyclostome Hox Clusters............................................. 121 Chapter 7 Hox Genes in Echinoderms.............................................................. 141 vii Get all Chapters For Ebook Instant Download by email at etutorsource@gmail.com We Don’t reply in this website, you need to contact by email for all chapters Instant download. Just send email and get all chapters download. Get all Chapters For Ebook Instant Download by email at etutorsource@gmail.com You can also order by WhatsApp https://api.whatsapp.com/send/?phone=%2B447507735190&text&type=ph one_number&app_absent=0 Send email or WhatsApp with complete Book title, Edition Number and Author Name. Get all Chapters For Ebook Instant Download by email at etutorsource@gmail.com viii Contents Chapter 8 Hox Genes in Mollusca..................................................................... 161 Chapter 9 The Evolution of Hox Genes in Spiralia........................................... 177 Index...................................................................................................................... 195 . Get all Chapters For Ebook Instant Download by email at etutorsource@gmail.com Get all Chapters For Ebook Instant Download by email at etutorsource@gmail.com 1 Introduction to Hox Modules in Evolution and Development CONTENTS 1.1 The Revolution of Evo-Devo............................................................................. 1 1.2 Homeotic Hox Homeobox Genes...................................................................... 2 1.3 A New Era of Wider Taxon-Sampling..............................................................4 1.4 Complex Regulatory Landscapes: Micromanagement and Collinearity........... 6 1.5 And There Is More............................................................................................ 8 1.6 Conclusion......................................................................................................... 8 References................................................................................................................... 9 1.1 THE REVOLUTION OF EVO-DEVO The Hox genes are a renowned and hugely important subset of developmental control genes in animals. The molecular characterisation of these genes began in the 1980s and utterly transformed developmental biology, as well as rejuvenating the field of evolutionary developmental biology (often abbreviated to evo-devo). A wealth of research has accumulated since the early days of molecular biology, with the Hox genes representing some of the best exemplars of the two sides of the evo-devo coin: deep homology versus diversification. This book aims to capture some of the latest developments in the field of Hox evo-devo research, illustrating the rich vein that these genes have provided for deepening our understanding of the origins and diversification of huge swathes of the animal kingdom. Hox genes are homeobox-containing genes; the homeobox is a distinctive nucleotide motif, typically of 180bp, that encodes a homeodomain, which in turn acts as a sequence-specific DNA-binding motif. Hox proteins thus tend to act as transcription factors. Few, if any, developmental gene pathways or regulatory networks do not involve homeobox genes. Although the Hox genes are most renowned for their roles in patterning the anterior-posterior axis of animals during embryogenesis (Akam, 1989; McGinnis and Krumlauf, 1992), the genes also have a multitude of activities and effects beyond this anterior-posterior patterning role, with complex regulatory landscapes facilitating activities in morphogenesis and organogenesis (Saurin et al., 1 Get all Chapters For Ebook Instant Download by email at etutorsource@gmail.com Get all Chapters For Ebook Instant Download by email at etutorsource@gmail.com 2 Hox Modules in Evolution and Development 2018; Hajirnis and Mishra, 2021). As such, the Hox genes have had a major role in the expansion and development of the field of evo-devo, following the discovery of the homeobox in the Hox genes of the fruit fly, Drosophila melanogaster, and then the rapid follow-on discoveries of conservation of the homeobox and homologous Hox genes across almost the entire animal kingdom, including in humans (McGinnis, 1984a, b; Scott and Weiner, 1984; Hart et al., 1985; Levine et al., 1985; Boncinelli et al., 1988; Duboule and Dollé, 1989; Graham et al., 1989; Gehring, 1994; Lewis, 1994; McGinnis, 1994). This widespread conservation of these homologous genes, their action in comparable and often homologous developmental processes, and their major roles in shaping animal body plans, with roles in generating both the similarities and differences between animal forms, has had a profound impact on biology and our understanding of the evolution of animal diversity. 1.2 HOMEOTIC HOX HOMEOBOX GENES The name homeobox derives from the phenomenon of homeosis described by William Bateson in his 1894 book Materials for the Study of Variation (reprinted as Bateson, 1992), for which the Hox genes of D. melanogaster are the best-known examples at the level of the genetics of such mutants. Homeosis entails one region or part of the body being transformed into another. One way to think of this is that things have not been deleted, duplicated, expanded or reduced, but instead the identity of something has changed such that its developmental fate becomes one that is usually located in a different place in the body plan. Some of the most famous homeotic mutants are Antennapedia, in which the antennae are transformed into legs (Gehring, 1966), or the Ubx four-winged fly (described in Chapter 2 by Karch and Maeda) in which the third thoracic segment instead develops with the identity of the wing-bearing second thoracic segment. The key feature of these types of mutants is that one region of the body is transformed such that it develops as another part of the body – i.e. it is homeotically transformed (Lewis, 1978, 1994). The genes effected by these mutations thus give particular regions or parts of the body their ‘identity’ and are entwined with the concept of positional information in embryogenesis, in this case in terms of a ‘Hox code’ – whereby a particular combination of Hox proteins specify the identity of a region of the body or a particular structure (Lewis, 1978; Wolpert, 1996). Thus, when the fly homeotic genes of the Antennapedia and Bithorax complexes (ANT-C and BX-C, respectively) were first cloned via the then pioneering techniques of genomic walking (Bender et al., 1983; Garber et al., 1983; Scott et al., 1983) and low stringency DNA hybridisation, and their DNA sequences revealed to have a conserved motif (McGinnis et al., 1984a, b; Scott and Weiner, 1984), it made perfect sense to call the motif the homeobox. Despite repeated use of the term ‘Hox’ in the literature interchangeably for homeobox, the Hox genes are in fact only a subset of homeoboxcontaining genes; being those genes that are orthologs of the genes of the fly and mouse/human Hox gene clusters (Scott, 1992; Ferrier, 2016a) (Figure 1.1A). Thus, whilst the human genome contains over 200 homeobox genes and arthropods like D. melanogaster over 100 (Holland et al., 2007; Chipman et al., 2014), there are only 39 human Hox genes and eight Hox genes in D. melanogaster (but with other Get all Chapters For Ebook Instant Download by email at etutorsource@gmail.com Get all Chapters For Ebook Instant Download by email at etutorsource@gmail.com Introduction to Hox Modules in Evolution and Development 3 FIGURE 1.1 The Hox gene clusters of the fly Drosophila melanogaster and human Homo sapiens, and a phylogenetic tree highlighting the relationships of the taxa discussed in this book. (A) The Hox clusters of the fly D. melanogaster and human, H. sapiens, highlighting the Hox genes and the Hox-derived genes (filled and empty triangles respectively), and non-homeobox genes (rounded rectangles) for the fly and the four human clusters resulting from the two rounds of Whole Genome Duplications (WGD) in early vertebrate evolution, followed by various gene loss events (represented as ‘missing’ triangles along the clusters). The different colours of the triangles represent the anterior, group 3, medial and posterior categories of Hox genes, and their orientation represents the direction of transcription for each gene. (B) A schematic phylogeny of selected taxa discussed in this book. See Chapter 9 by Gasiorowski, Martín-Durán and Hejnol for further elaboration of the spiralian clade. Get all Chapters For Ebook Instant Download by email at etutorsource@gmail.com Get all Chapters For Ebook Instant Download by email at etutorsource@gmail.com 4 Hox Modules in Evolution and Development homeobox-containing genes within the ANT-C part of the Hox cluster derived from bona fide Hox genes, i.e. zerknüllt 1 and 2 and bicoid from Hox 3, and fushi tarazu from a medial Hox gene perhaps with affinity to Lox5 or Hox6 of lophotrochozoans or deuterostomes, respectively; Falciani et al., 1996; Telford, 2000) (Figure 1.1A). It is these Hox genes and their orthologs across the animal kingdom that this book focuses on (Figure 1.1B). 1.3 A NEW ERA OF WIDER TAXON-SAMPLING Our understanding of the function and regulation of Hox genes in Drosophila is resolved to unprecedented detail, stemming from the elegant and thorough work in genetics by Ed Lewis and colleagues, and flourishing still further with the impact of developments in molecular techniques for the BX-C (as outlined by Karch and Maeda in Chapter 2), and in parallel for the ANT-C (see (Denell, 1994) for a perspective on the early genetic studies of the ANT-C, which were later expanded by the Kaufman lab and many others). This detailed knowledge of Drosophila is complemented by the elegant and detailed work in vertebrates, particularly in mice (e.g. Kmita and Duboule, 2003; Montavon et al., 2011; Nolte et al., 2013; Ahn et al., 2014; Lonfat et al., 2014; Darbellay et al., 2019; Amandio et al., 2021). Whilst there is clearly much more to be understood about Hox genes and their activities in Drosophila and mice, the extent of the similarities and differences between these so-called model species to other taxa is still largely an unresolved mystery. However, we are now entering an era in which this mystery can be clarified, with techniques becoming available that permit efficient exploration of molecular mechanisms without requiring the heroic efforts such as those exerted by Ed Lewis in unravelling the genetics of a species, so that there is the possibility of exploring taxa largely driven by their phylogenetic position or mode of development, instead of their amenability to genetic approaches. The early description of the Hox genes as the Rosetta stone for developmental biology (Slack, 1984) is even more apposite now than ever, with the genes providing a rapid means to delve into the molecular mechanisms of how animal form is constructed and how it evolved. This book thus comes at a time when the field is rich with possibilities, and the authors here give a clear overview of where the field is going. Starting from the detailed mechanistic understanding of Hox gene regulation and function that can be obtained in more ‘traditional’ study species like D. mela­ nogaster (see Karch and Maeda, Chapter 2), the power of wider taxon sampling is clearly demonstrated in subsequent chapters. Moving progressively further away from Drosophila, phylogenetically speaking, Zattara and Moczek (Chapter 3) use other insects, the horn-bearing beetles, as a system to understand the evo-devo of novel morphologies and evolvability – long-standing issues and debates in evolutionary biology that are now getting a new perspective from evo-devo and Hox studies in particular. Beyond insects, Sharma (Chapter 4) uses the chelicerate arthropods (i.e. spiders, mites, sea spiders and horseshoe crabs) to study the impacts of whole genome duplication (WGD) and the importance of understanding the underlying species phylogeny (which Hox genes can contribute to) in order to make accurate and appropriate evolutionary inferences. Get all Chapters For Ebook Instant Download by email at etutorsource@gmail.com We Don’t reply in this website, you need to contact by email for all chapters Instant download. Just send email and get all chapters download. Get all Chapters For Ebook Instant Download by email at etutorsource@gmail.com You can also order by WhatsApp https://api.whatsapp.com/send/?phone=%2B447507735190&text&type=ph one_number&app_absent=0 Send email or WhatsApp with complete Book title, Edition Number and Author Name. Get all Chapters For Ebook Instant Download by email at etutorsource@gmail.com Introduction to Hox Modules in Evolution and Development 5 These chelicerate WGDs are an intriguing case of a clade that has experienced WGDs that have been a key factor in shaping the structure, organisation and content of these genomes, with processes such as duplication-degeneration-complementation (DDC) (Force et al., 1999) impacting the evolution of the regulation and function of the developmental control genes (e.g. Schwager et al., 2017; Leite et al., 2018). Hox genes have been and are proving to be key study systems to better understand the impacts of gene duplication, via processes such as WGD, as a route to increased genetic diversity and its interplay with developmental and morphological evolution. The more well-known instance of WGD shaping the genomes of a large clade is the case of the vertebrates. It is now well-established that the genomes of the gnathostome (jawed) vertebrates were shaped by two rounds of WGD (the 2R hypothesis) (Lamb, 2021; Nakatani et al., 2021), with further instances of additional WGD in lineages such as the teleosts (i.e. the teleost 3R WGD; Amores et al., 1998; Taylor et al., 2001; Aase-Remedios and Ferrier, 2021). Resolution of these 2R and 3R events in gnathostomes has been significantly improved with non-teleost data such as that from chondrichthyans (see Kuraku, Chapter 5), which now reveals the dynamics of post-2R Hox evolution to unprecedented detail, including gene and cluster losses in distinct lineages and unusual or distinctive divergences (e.g. HoxC genes), as well as cryptic pan-vertebrate genes like Hox14 (Kuraku et al., 2016). Furthermore, a major area of debate with regards to the 2R vertebrate events is how do the jawless cyclostome vertebrates (or agnathans) fit into the picture. Are they also descended from the same 2R events that shaped the gnathostomes, or not? And if not, then did they experience just one of the 2R events (i.e. cyclostomes diverged after 1R) or neither of the 2R WGDs? Clearly, there are large numbers of paralogs in cyclostome genomes and so they certainly did undergo large-scale duplications of some sort, but the difficulties that have been experienced over the years in reliably placing cyclostome genes into molecular phylogenetic trees have contributed to a diversity of opinions on how to interpret the data. The chapter of Pascual-Anaya and Böhmer (Chapter 6) gives an update on the cyclostome field and the prominent role that Hox genes have played in it. They provide a new hypothesis for the state of the last common ancestor of vertebrates in terms of the array of Hox gene clusters that it possessed. If we move deeper into the deuterostomes, beyond the vertebrates, we encounter one of the most unusual body plans in the animal kingdom: that of the pentameral echinoderms. Given the key role that the Hox genes play in patterning the anterior-­ posterior axis of bilaterian animals, many in the field of echinoderm evo-devo have been focused on how these genes might pattern the pentameral body plan and hence how such a novel morphology evolved. Omori and Irie (Chapter 7) provide an overview of the latest echinoderm Hox research, demonstrating the clear value of wide taxon sampling and extending beyond what has historically been the major echinoderm study system of the purple sea urchin, Strongylocentrotus purpuratus, to obtain a clear picture of what constitutes the echinoderm Hox complement and organisation and how these genes are deployed during echinoderm development. Data from such a wide selection of species, sampling from all of the extant echinoderm classes, has clearly been essential to construct a more reliable picture of the ancestral state for this intriguing phylum and facilitate more robust comparisons to other bilaterian phyla. Wide taxon sampling is key. Get all Chapters For Ebook Instant Download by email at etutorsource@gmail.com Get all Chapters For Ebook Instant Download by email at etutorsource@gmail.com 6 Hox Modules in Evolution and Development Until relatively recently, a major part of the animal kingdom was largely neglected in molecular evo-devo research, i.e. the phyla of the Lophotrochozoa/Spiralia. Perhaps this was in large part due to the traditional study species in developmental biology being from the Ecdysozoa and Deuterostomia (i.e. D. melanogaster as an ecdysozoan and vertebrates like mice and zebrafish being deuterostomes). This relative neglect is now being rectified, as demonstrated by the chapters by Wollesen and Wanninger, and Gasiorowski and colleagues (Chapters 8 and 9 respectively). As well as providing insights into some of the most morphologically diverse phyla, such as the molluscs, and the roles of Hox genes in shaping these body plans and the novelties they contain, this lophotrochozoan data is also proving valuable in shaping our views on the links between Hox cluster organisation and the regulation of the genes. Co-option as a major process in evo-devo (True and Carroll, 2002) is a common theme in the Hox genes of these lophotrochozoan taxa, with Hox genes frequently being redeployed from their pleisiomorphic ancestral roles in axial patterning into apomorphies or evolutionary novelties. Pending functional genetic data, it is presumed that the Hox genes have an integral role in controlling at least some aspect of the development of these evolutionary novelties. Thus, we come full circle in the chapters of this book, with Hox roles in anterior-posterior patterning in insects like the fruit fly alongside co-option into the evolution of novelties like beetle horns, to Hox roles in both axial patterning and development of novelties in lophotrochozoans like the molluscs, largely via the evolution of the regulation of the Hox genes. 1.4 COMPLEX REGULATORY LANDSCAPES: MICROMANAGEMENT AND COLLINEARITY One of the early stumbling blocks to the widespread adoption of ideas on roles for Hox gene changes in evolution was the perception that mutation of these genes would always have dramatic impacts, usually early in embryogenesis, that would lead to large changes that would almost always be detrimental (akin to the idea of hopeful monsters attributed to Goldschmidt, 1940). With the current much deeper understanding of the complexities and modularity of the regulation of the Hox genes and their roles in development, it is clear that we no longer have to hypothesise hopeful monsters. Instead, Hox genes can be viewed as micro-managers rather than master control genes – hopeful monsters are not necessarily impossible, but are certainly not required for Hox changes to have significant roles in evo-devo (Akam, 1998a, b). The micro-manager idea was stimulated by research on Hox gene regulation and pleiotropy, emphasising the importance both of modular regulatory elements and the highly dynamic expression of Hox genes in time and space, as well as the abundance of target genes, all of which can accumulate multiple small changes over the vast periods of evolutionary time to sum to apparently saltational changes when comparing extant taxa that diverged many millions of years ago (Akam, 1998a, b; Buffry and McGregor, 2022). In time, the detailed understanding of Hox gene regulation and function that we possess for D. melanogaster will be extended across the animal kingdom, providing even greater explanatory power to Hox genes and evo-devo. Hox gene regulation also provides one of the major phenomena that the Hox genes are renowned for: Collinearity. This entails the order of the genes along the Get all Chapters For Ebook Instant Download by email at etutorsource@gmail.com Get all Chapters For Ebook Instant Download by email at etutorsource@gmail.com Introduction to Hox Modules in Evolution and Development 7 chromosome corresponding to the order that the genes are expressed along the anterior-posterior axis, both in terms of their domains of expression (spatial collinearity) and the sequence in which they are activated (temporal collinearity – with anterior acting genes expressed first, through to posterior genes being activated last) (Von Allmen et al., 1996; Ferrier and Minguillón, 2003; Deschamps and Duboule, 2017; Krumlauf, 2018; Duboule, 2022). Nevertheless, it is clear that there are many cases of Hox genes not being clustered (Lemons and McGinnis, 2006; Monteiro and Ferrier, 2006), and a range of types of Hox gene organisation are recognised (Duboule, 2007). A potentially unifying principle behind the organisation of the Hox genes presumably relates to how these genes are regulated, following the abundant evidence for various long-range regulatory processes and phenomena such as topologically associating domains (TADs) (Acemel et al., 2016; Duboule, 2022), shared enhancers (Sharpe et al., 1998; Kmita and Duboule, 2003; Ahn et al., 2014; Miller and Posakony, 2020; and see Chapter 2 by Karch and Maeda), intermingled enhancers (Shippy et al., 2008), chromatin modulation (Chambeyron and Bickmore, 2004; Chambeyron et al., 2005), insulator/boundary elements and collinearity of enhancers (see Chapter 2 by Karch and Maeda), all of these things creating regulatory landscapes over Hox clusters at least in some taxa. From views on collinearity being focused on the overall spatial and temporal activation of the genes, with the pre-­eminence of temporal collinearity being viewed as the major process or mechanism(s) constraining the Hox genes in intact, ordered clusters in the genome (e.g. Duboule, 1994; Von Allmen et al., 1996; Ferrier and Holland, 2002; Ferrier and Minguillón, 2003; Monteiro and Ferrier, 2006; Deschamps and Duboule, 2017; Pascual-Anaya et al., 2018; Duboule, 2022), the lophotrochozoan data has been responsible for stimulating more nuanced views, such as sub-cluster temporal collinearity (Wang et al., 2017; Ferrier, 2019; see Chapter 9 by Gasiorowski et al.). Once again wider taxon sampling has been key. We are still quite some way from having a clear overview of collinearity, in terms of what it actually constitutes, how it is achieved, and the diversity or universality of mechanisms and processes that are under its umbrella. But we now have a clearer prospect for resolving the issue(s) than ever before, with the rapid developments in genome sequencing and functional genetics, all driven by the essential requirement for wide taxon sampling, as clearly demonstrated in this book. This will go handin-hand with the continued development and refinement of the key techniques that are integral to this science. Functional genetics in diverse species that do not have a history of or amenability to classical genetic approaches was opened up by the various approaches to RNA interference (RNAi) and is now experiencing a further revolution with the application of the CRISPR/Cas technique (see the discussions in Chapters 3 and 4 by Zattarra and Moczek, and Sharma, respectively). Genome and transcriptome sequencing became more affordable, and hence feasible, with the advent of so-called next-gen sequencing, and these rapid technical developments have continued with the invention and wide application of such techniques as longmolecule sequencing and Hi-C sequencing to produce much better genome sequence assemblies, often now to the level of whole chromosome resolution. This degree of resolution is essential for robustly identifying linkage and clustering of genes like the Hox genes and the genomic context that they evolved and function in (Pollard Get all Chapters For Ebook Instant Download by email at etutorsource@gmail.com Get all Chapters For Ebook Instant Download by email at etutorsource@gmail.com 8 Hox Modules in Evolution and Development and Holland, 2000; Garcia-Fernàndez, 2005; Butts et al., 2008; Ferrier, 2016a). Understanding the significance of Hox gene organisation goes hand-in-hand with understanding how the genes are expressed (in time and space) and what developmental functions this expression is entwined with. This is exemplified throughout the chapters of this book. 1.5 AND THERE IS MORE We have not been able to capture all of the research that is ongoing in the area of Hox gene evo-devo and could easily fill another book (or more) with Hox research in other taxa, impacting further evolutionary and developmental concepts and biological processes. We could have included other parts of the animal kingdom, such as non-­bilaterians (including sea anemones and jellyfish) to help understand the evolutionary transition between the diploblasts and triploblasts (or radially symmetrical and bilaterian animals, depending on one’s perspective) (DuBuc et al., 2018; He et al., 2018). Or even more basal lineages in the animal phylogeny, such as sponges (Porifera) and comb jellies (Ctenophora) to understand the origin of the Hox genes themselves, relative to other homeobox gene families (Mendivil Ramos et al., 2012; Fortunato et al., 2014; Ferrier, 2016b). Also, the gnathostome vertebrates (such as the various jawed fish) have been the source for important research on Hox paralog evolution (or ohnolog evolution – ohnolog being the name given to paralogs produced from WGD, in tribute to Susumo Ohno who first hypothesised a major role for WGD in chordate evolution; Ohno, 1970; Wolfe, 2000) and morphological innovations such as the paired appendages and digits, appendage positions, or varied vertebral transitions, to name but a few (Burke et al., 1995; Mallo, 2018; Moreau et al., 2019; Meyer et al., 2021). We could also have explored the diversity of examples of evolution via changes to Hox regulation versus changes to protein-coding sequences, as a major route to exploring the debates about the relative balance of such changes in evo-devo and how the gene regulatory networks (GRNs) containing Hox genes evolve (e.g. Greer et al., 2000; Ronshaugen et al., 2002; Liu et al., 2019; Allais-Bonnet et al., 2021; Lynch and Wagner, 2021). Also on a regulatory theme, research on the Hox cluster of D. melanogaster was pioneering in discovering activities of non-coding RNA genes (Sànchez-Herrero and Akam, 1989), which has blossomed into roles for microRNAs, long non-coding RNAs and antisense RNA transcripts, with Hox examples often leading the way for our understanding of how these ‘RNA’ genes work (De Kumar and Krumlauf, 2016) (also see Chapters 2 and 5 by Karch and Maeda, and Kuraku, respectively). This book is clearly to be viewed as a selective sample of Hox evo-devo research. 1.6 CONCLUSION A core theme of this book and Hox research is the comparative method. The power of the comparative method is intrinsic to understanding fundamental aspects of biology, such as pattern formation, cell fate determination, morphogenesis, GRNs, homology and the origins of form in both ontogenesis and evolutionary time, and so Get all Chapters For Ebook Instant Download by email at etutorsource@gmail.com Get all Chapters For Ebook Instant Download by email at etutorsource@gmail.com Introduction to Hox Modules in Evolution and Development 9 how evolution has produced the incredible biodiversity that we see around us today (as well as everything that has existed in the past and is now extinct). The scope and power of this comparative method combined with the revolution of Hox genes cannot be underestimated. Hox genes are now firmly established as fundamental elements in two major traditional branches of biology, Developmental Biology and Evolutionary Biology, and offer one of the most fertile and powerful integrated overlaps of the two, which is now firmly established as the field of Evo-Devo. Hox gene research has been truly revolutionary and shows every indication of continuing to be so. REFERENCES Aase-Remedios, M.E. and D.E.K. Ferrier. 2021. Improved understanding of the role of gene and genome duplications in chordate evolution with new genome and transcriptome sequences. Frontiers in Ecology and Evolution 9: 703163. Acemel, R.D., J.J. Tena, I. Irastorza-Azcarate, et al. 2016. A single three-dimensional chromatin compartment in amphioxus indicates a stepwise evolution of vertebrate Hox bimodal regulation. Nature Genetics 48: 336–341. Ahn, Y., H.E. Mullan and R. Krumlauf. 2014. Long-range regulation by shared retinoic acid response elements modulates dynamic expression of posterior Hoxb genes in CNS development. Developmental Biology 388: 134–144. Akam, M. 1989. Hox and HOM: homologous gene clusters in insects and vertebrates. Cell 57: 347–349. Akam, M. 1998a. Hox genes: from master genes to micromanagers. Current Biology 8: R676–R678. Akam, M. 1998b. Hox genes, homeosis and the evolution of segment identity: no need for hopeless monsters. International Journal of Development Biology 42: 445–451. Allais-Bonnet, A., A. Hintermann, M.-C. Deloche, et al. 2021. Analysis of polycerate mutants reveals the evolutionary co-option of HOXD1 for horn patterning in Bovidae. Molecular Biology and Evolution 38: 2260–2272. Amandio, A.R., L. Beccari, L. Lopez-Delisle, B. Mascrez, J. Zakany, S. Gitto and D. Duboule. 2021. Sequential in cis mutagenesis in vivo reveals various functions for CTCF sites at the mouse HoxD cluster. Genes & Development 35: 1490–1509. Amores, A., A. Force, Y.L. Yan, L. Joly, C. Amemiya, A. Fritz, R.K. Ho, J. Langeland, V. Prince, Y.L. Wang, M. Westerfield, M. Ekker and J.H. Postlethwait. 1998. Zebrafish hox clusters and vertebrate genome evolution. Science 282: 1711–1714. Bateson, W. 1992. Materials for the study of variation: treated with especial regard to dis­ continuity in the origin of species. Baltimore, John Hopkins University Press. Bender, W., M. Akam, F. Karch, P.A. Beachy, M. Peifer, P. Spierer, E.B. Lewis and D.S. Hogness. 1983. Molecular genetics of the bithorax complex in Drosophila melanogas­ ter. Science 221: 23–29. Boncinelli, E., R. Somma, D. Acampora, et al. 1988. Organization of human homeobox genes. Human Reproduction 3: 880–886. Buffry, A.D. and A.P. McGregor. 2022. Micromanagement of Drosophila post-embryonic development by Hox genes. Journal of Developmental Biology 10: 13. Burke, A.C., C.E. Nelson, B.A. Morgan and C. Tabin. 1995. Hox genes and the evolution of vertebrate axial morphology. Development 121: 333–346. Butts, T., P.W.H. Holland and D.E.K. Ferrier. 2008. The urbilaterian super-Hox cluster. Trends in Genetics 24: 259–262. Chambeyron, S. and W.A. Bickmore. 2004. Chromatin decondensation and nuclear reorganization of the HoxB locus upon induction of transcription. Genes & Development 18: 1119–1130. Get all Chapters For Ebook Instant Download by email at etutorsource@gmail.com We Don’t reply in this website, you need to contact by email for all chapters Instant download. Just send email and get all chapters download. Get all Chapters For Ebook Instant Download by email at etutorsource@gmail.com You can also order by WhatsApp https://api.whatsapp.com/send/?phone=%2B447507735190&text&type=ph one_number&app_absent=0 Send email or WhatsApp with complete Book title, Edition Number and Author Name. Get all Chapters For Ebook Instant Download by email at etutorsource@gmail.com 10 Hox Modules in Evolution and Development Chambeyron, S., N.R. Da Silva, K.A. Lawson and W.A. Bickmore. 2005. Nuclear re-­ organisation of the Hoxb complex during mouse embryonic development. Development 132: 2215–2223. Chipman, A.D., D.E.K Ferrier. C. Brena, et al. 2014. The first myriapod genome sequence reveals conservative arthropod gene content and genome organisation in the centipede Strigamia maritima. PLOS Biology 12: e1002005. Darbellay, F., C. Bochaton, L. Lopez-Delisle, B. Mascrez, P. Tschopp, S. Delpretti, J. Zakany and D. Duboule. 2019. The constrained architecture of mammalian Hox gene clusters. Proceedings of the National Academy of the Sciences of the United States of America 116: 13424–13433. De Kumar, B. and R. Krumlauf. 2016. HOXs and lincRNAs: two sides of the same coin. Science Advances 2: e1501402. Denell, R. 1994. Discovery and genetic definition of the Drosophila antennapedia complex. Genetics 138: 549–552. Deschamps, J. and D. Duboule. 2017. Embryonic timing, axial stem cells, chromatin dynamics, and the Hox clock. Genes Development 31: 1406–1416. Duboule, D. 1994. Temporal colinearity and the phylotypic progression: a basis for the stability of a vertebrate Bauplan and the evolution of morphologies through heterochrony. Development (1994 Supplement) 135–142. Duboule, D. 2007. The rise and fall of Hox gene clusters. Development 134: 2549–2560. Duboule, D. 2022. The (unusual) heuristic value of Hox gene clusters: a matter of time? Developmental Biology 484: 75–87. Duboule, D. and P. Dollé. 1989. The structural and functional organization of the murine HOX gene family resembles that of Drosophila homeotic genes. EMBO Journal 8: 1497–1505. DuBuc, T.Q., T.B. Stephenson, A.Q. Rock and M.Q. Martindale. 2018. Hox and Wnt pattern the primary body axis of an anthozoan cnidarian before gastrulation. Nature Communications 9: 2007. Falciani, F., B. Hausdorf, R. Schröder, M. Akam, D. Tautz, R. Denell and S. Brown. 1996. Class 3 Hox genes in insects and the origin of zen. Proceedings of the National Academy of the Sciences of the United States of America 93: 8479–8484. Ferrier, D.E.K. 2016a. Evolution of homeobox gene clusters in animals: the Giga-cluster and primary versus secondary clustering. Frontiers in Ecology and Evolution 4: 36. Ferrier, D.E.K. 2016b. The origin of the Hox/ParaHox genes, the Ghost Locus hypothesis and the complexity of the first animal. Briefings in Functional Genomics 15: 333–341. Ferrier, D.E.K. 2019. Space and time in Hox/ParaHox gene cluster evolution. In Perspectives on evolutionary and developmental biology: essays for Alessandro Minelli, ed. G. Fusco, 245–258. Padova, Padova University Press. Ferrier, D.E.K. and C. Minguillón. 2003. Evolution of the Hox/ParaHox gene clusters. International Journal of Developmental Biology 47: 605–611. Ferrier, D.E.K. and P.W.H. Holland. 2002. Ciona intestinalis ParaHox genes: evolution of Hox/ParaHox cluster integrity, developmental mode, and temporal colinearity. Molecular Phylogenetics and Evolution 24: 412–417. Force, A., M. Lynch, F. B. Pickett, A. Amores, Y. Yan and J. Postlethwait. 1999. Preservation of duplicate genes by complementary, degenerative mutations. Genetics 151: 1531–1545. Fortunato, S.A.V., M. Adamski, O. Mendivil Ramos, S. Leininger, J. Liu, D.E.K. Ferrier and M. Adamska. 2014. Calcisponges have a ParaHox gene and dynamic expression of dispersed NK homeobox genes. Nature 514: 620–623. Garber, R.L., A. Kuroiwa and W.J. Gehring. 1983. Genomic and cDNA clones of the homeotic locus Antennapedia in Drosophila. EMBO Journal 2: 2027–2036. Garcia-Fernàndez, J. 2005. The genesis and evolution of homeobox gene clusters. Nature Reviews Genetics 6: 881–892. Get all Chapters For Ebook Instant Download by email at etutorsource@gmail.com Get all Chapters For Ebook Instant Download by email at etutorsource@gmail.com Introduction to Hox Modules in Evolution and Development 11 Gehring, W. 1966. Bildung eines vollstaendigen Mittelbeins mit Sternopleural in der Antennenregion bei der Mutante Nasobemia (Ns) von Drosophila melanogaster. Arch Julius Klaus Stift Vererbungsforsch Sozialanthropol Rassenhyg 41: 44–54. Gehring, W.J. 1994. A history of the homeobox. In Guidebook to the homeobox genes, ed. D. Duboule, 3–10. Oxford, Sambrook and Tooze Publishing Partnership, Oxford University Press. Goldschmidt, R. 1940. The material basis of evolution. New Haven, Yale University Press. Graham, A., N. Papalopulu and R. Krumlauf. 1989. The murine and Drosophila homeobox gene complexes have common features of organization and expression. Cell 57: 367–378. Greer, J.M., J. Puetz, K.R. Thomas and M.R. Capecchi. 2000. Maintenance of functional equivalence during paralogous Hox gene evolution. Nature 403: 661–665. Hajirnis, N. and R.K. Mishra. 2021. Homeotic genes: clustering, modularity, and diversity. Frontiers in Cell and Developmental Biology 9: 718308. Hart, C.P., A. Awgulewitsch, A. Fainsod, W. McGinnis and F.H. Ruddle. 1985. Homeobox gene complex on mouse chromosome 11: molecular cloning, expression in embryogenesis, and homology to a human homeobox locus. Cell 43: 9–18. He, S., F. Del Viso, C.-Y. Chen, A. Ikmi, A.E. Kroesen and M.C. Gibson. 2018. An axial Hox code controls tissue segmentation and body patterning in Nematostella vectensis. Science 361: 1377–1380. Holland, P.W.H., H.A. Booth and E.A. Bruford. 2007. Classification and nomenclature of all human homeobox genes. BMC Biology 5: 47. Kmita, M. and D. Duboule. 2003. Organizing axes in time and space; 25 years of colinear tinkering. Science 301: 331–333. Krumlauf, R. 2018. Hox genes, clusters and colinearity. International Journal of Develop­ mental Biology 62: 659–663. Kuraku, S., N. Feiner, S.D. Keeley and Y. Hara. 2016. Incorporating tree-thinking and evolutionary time scale into developmental biology. Development, Growth & Differentiation 58, 131–142. Lamb, T.D. 2021. Analysis of paralogons, origin of the vertebrate karyotype, and ancient chromosomes retained in extant species. Genome Biology and Evolution 13: evab044. Leite, D.J., L. Baudouin-Gonzalez, S. Iwasaki-Yokozawa, et al. 2018. Homeobox gene duplication and divergence in arachnids. Molecular Biology and Evolution 35: 2240–2253. Lemons, D. and W. McGinnis. 2006. Genomic evolution of Hox gene clusters. Science 313: 1918–1922. Levine, M., G. Rubin, and R. Tijan. 1985. Human DNA sequences homologous to a protein coding region conserved between homeotic genes of Drosophila. Cell 38: 667–673. Lewis, E.B. 1978. A gene complex controlling segmentation in Drosophila. Nature 276: 565–570. Lewis, E.B. 1994. Homeosis: the first 100 years. Trends in Genetics 10: 341–343. Liu, Y., M. Ramos-Womack, C. Han, et al. 2019. Changes throughout a genetic network mask the contribution of Hox gene evolution. Current Biology 29: 2157–2166. Lonfat, N., T. Montavon, F. Darbellay, S. Gitto and D. Duboule. 2014. Convergent evolution of complex regulatory landscapes and pleiotropy at Hox loci. Science 346: 1004–1006. Lynch, V.J. and Wagner, G.P. 2021. Cooption of polyalanine tract into a repressor domain in the mammalian transcription factor HoxA11. Journal of Experimental Zoology Part B: Molecular and Developmental Evolution 1–10. DOI: 10.1002/jez.b.23063 Mallo, M. 2018. Reassessing the role of Hox genes during vertebrate development and evolution. Trends Genetics 43: 209–217. McGinnis, W. 1994. A century of homeosis, a decade of homeoboxes. Genetics 137: 607–611. McGinnis, W. and R. Krumlauf. 1992. Homeobox genes and axial patterning. Cell 68: 283–302. Get all Chapters For Ebook Instant Download by email at etutorsource@gmail.com Get all Chapters For Ebook Instant Download by email at etutorsource@gmail.com 12 Hox Modules in Evolution and Development McGinnis, W., M. Levine, E. Hafen, A. Kuroiwa and W.J. Gehring. 1984a. A conserved DNA sequence found in homeotic genes of the Drosophila antennapedia and Bithorax complexes. Nature 308: 428–433. McGinnis. W., C.P. Hart, W.J. Gehring, and F.H. Ruddle. 1984b. Molecular cloning and chromosome mapping of a mouse DNA sequence homologous to homeotic genes of Drosophila. Cell 38: 675–680. McGinnis. W., R.L. Garber, J. Wirz, A. Kuroiwa and W.J. Gehring. 1985b. A homologous protein-coding sequence in Drosophila homeotic genes and its conservation in other metazoans. Cell 37: 403–408. Mendivil Ramos, O., D. Barker and D.E.K. Ferrier. 2012. Ghost loci imply Hox and ParaHox existence in the last common ancestor of animals. Current Biology 22: 1951–1956. Meyer, A., S. Schloissnig, P. Franchini, et al. 2021. Giant lungfish genome elucidates the conquest of land by vertebrates. Nature 590: 284–289. Miller, S.W. and J.W. Posakony. 2020. Disparate expression specificities coded by a shared Hox-C enhancer. eLife 9: e39876. Montavon, T., N. Soshnikova, B. Mascrez, E. Joye, L. Thevenet, E. Splinter, W. de Laat, F. Spitz and D. Duboule. 2011. A regulatory archipelago controls Hox genes transcription in digits. Cell 147: 1132–1145. Monteiro, A.S. and D.E.K. Ferrier. 2006. Hox genes are not always Colinear. International Journal of Biological Sciences 2: 95–103. Moreau, C., P. Caldarelli, D. Rocancourt, J. Roussel, N. Denans, O. Pourquie and J. Gros. 2019. Timed collinear activation of Hox genes during gastrulation controls the avian forelimb position. Current Biology 29: 35–50. Nakatani, Y., P. Shingate, V. Ravi, N.E. Pillai, A. Prasad, A. McLysaght and B. Venkatesh. 2021. Reconstruction of proto-vertebrate, proto-cyclostome and proto-gnathostome genomes provides new insights into early vertebrate evolution. Nature Communications 12: 4489. Nolte, C., T. Jinks, X. Wang, M.T. Martinez Pastor and R. Krumlauf. 2013. Shadow enhancers flanking the HoxB cluster direct dynamic Hox expression in early heart and endoderm development. Developmental Biology 383: 158–173. Ohno, S. 1970. Evolution by gene duplication. London, George Allen and Unwin. Pascual-Anaya, J., I. Sato, F. Sugahara, et al. 2018. Hagfish and lamprey Hox genes reveal conservation of temporal collinearity in vertebrates. Nature Ecology and Evolution 2: 859–866. Pollard, S.L. and P.W.H. Holland. 2000. Evidence for 14 homeobox gene clusters in human genome ancestry. Current Biology 10: 1059–1062. Ronshaugen, M., N. McGinnis and W. McGinnis. 2002. Hox protein mutation and macroevolution of the insect body plan. Nature 415: 914–917. Sànchez-Herrero, E. and M.E. Akam. 1989. Spatially ordered transcription of regulatory DNA in the bithorax complex of Drosophila. Development 107: 321–329. Saurin, A.J., M.C. Delfini, C. Maurel-Zaffran and Y. Graba. 2018. The generic facet of Hox protein function. Trends in Genetics 34: 941–953. Schwager, E.E., P.P. Sharma, T. Clarke, et al. 2017. The house spider genome reveals an ancient whole-genome duplication during arachnid evolution. BMC Biology 15: 62. Scott, M.P. 1992. Vertebrate homeobox gene nomenclature. Cell 71: 551–553. Scott, M.P., A.J. Weiner, T.I. Hazelrigg, B.A. Polisky, V. Pirrotta, F. Scalenghe and T.C. Kaufman. 1983. The molecular organization of the Antennapedia locus of Drosophila. Cell 35: 763–776. Scott, M.P. and A. Weiner. 1984. Structural relationships among genes that control development: sequence homology between the Antennapedia, Ultrabithorax, and Fushi Tarazu loci of Drosophila. Proceedings of the National Academy of Sciences of the United States of America 81: 4115–4119. Get all Chapters For Ebook Instant Download by email at etutorsource@gmail.com Get all Chapters For Ebook Instant Download by email at etutorsource@gmail.com Introduction to Hox Modules in Evolution and Development 13 Sharpe, J., S. Nonchev, A. Gould, J. Whiting and R. Krumlauf. 1998. Selectivity, sharing and competitive interactions in the regulation of Hoxb genes. EMBO J. 17: 1788–1798. Shippy, T.D., M. Ronshaugen, J. Cande, J.P. He, R.W. Beeman, M. Levine, S.J. Brown and R.E. Denell. 2008. Analysis of the Tribolium homeotic complex: insights into mechanisms constraining insect Hox clusters. Development Genes and Evolution 218: 127–139. Slack, J. 1984. Developmental biology: a Rosetta stone for pattern formation in animals? Nature 310: 364–365. Taylor, J.S., Y. Van de Peer, I. Braasch and A. Meyer. 2001. Comparative genomics provides evidence for an ancient genome duplication event in fish. Philosophical Transactions of the Royal Society B: Biological Sciences 356: 1661–1679. Telford, M.J. 2000. Evidence for the derivation of the Drosophila fushi tarazu gene from a Hox gene orthologous to lophotrochozoan Lox5. Current Biology 10: 349–352. True, J. and S.B. Carroll. 2002. Gene co-option in physiological and morphological evolution. Annual Review of Cell and Developmental Biology 18: 53–80. Von Allmen, G., I. Hogga, A. Spierer, F. Karch, W. Bender, H. Gyurkovics and E. Lewis. 1996. Splits in fruitfly Hox gene complexes. Nature 380: 116. Wang, S., J. Zhang, W. Jiao, et al. 2017. Scallop genome provides insights into evolution of bilaterian karyotype and development. Nature Ecology and Evolution 1: 120. Wolfe, K. 2000. Robustness – it’s not where you think it is. Nature Genetics 25: 3–4. Wolpert, L. 1996. One hundred years of positional information. Trends in Genetics 12: 359–364. Get all Chapters For Ebook Instant Download by email at etutorsource@gmail.com We Don’t reply in this website, you need to contact by email for all chapters Instant download. Just send email and get all chapters download. Get all Chapters For Ebook Instant Download by email at etutorsource@gmail.com You can also order by WhatsApp https://api.whatsapp.com/send/?phone=%2B447507735190&text&type=ph one_number&app_absent=0 Send email or WhatsApp with complete Book title, Edition Number and Author Name.