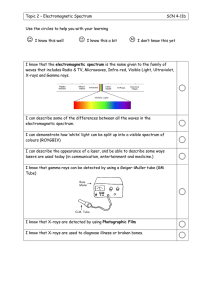
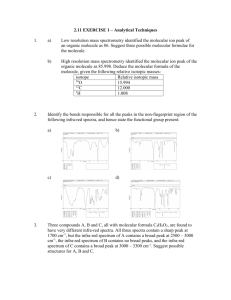
INFRA-RED SPECTROSCOPY NOTES AS-AL CHEMISTRY 2021-22 Infrared spectroscopy is used to identify functional groups in organic molecules. Most compounds absorb i When a sample of an organic compound is irradiated with infra-red frequencies, you find that the compoun A detector would show that some frequencies pass through the compound with almost no loss, but other fr What causes some frequencies to be absorbed? Each frequency of infra-red has a certain energy. If a particular frequency is being absorbed as it passes th Energies in infra-red radiation correspond to the energies involved in bond vibrations. Bonds are vibrating all the time, but if you irradiate exactly the right amount of energy on a bond, you can m The position of a peak depends on: Bond strength Masses of the atoms joined by the bond Strong bonds and light atoms absorb at higher wave numbers Weak bonds and heavy atoms absorb at lower wave numbers Finger print Region The region to the right-hand side of the spectra (from about 1500 to 500 cm-1) usually contains a very com INTERPRETING AN INFRA-RED SPECTRUM The infra-red spectrum for an alcohol It’s the absorption of infra-red radiation by atmospheric gases such as methane, carbon dioxide and water By measuring the characteristic wavelengths of infrared radiation absorbed by the molecules of the polluta They can also analyze the intensity of the absorptions to find the concentration of each pollutant present in