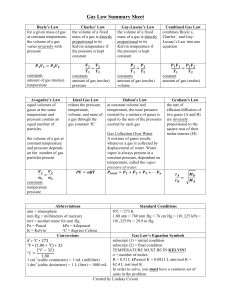
Gases We have talked some about solids and liquids, now some about gases. Pressure A gas uniformly fills any container, is easily compressed, and mixes completely with any other gas. It also exerts pressure (P=F/A) Barometers measure pressure A mercury barometer is a thin tube filled with Hg and then inverted into a bath of more Hg. At sea level the Hg will stay in the tube to a measurement of 760 mm high. Evangelista Torricelli (15 October 1608 – 25 October 1647) Another way to measure the pressure of a sample of gas is with a manometer Chemistry 121 Notes 16 – Gases Page 1 of 7 Units of Pressure Because we use Hg normally to measure pressure we use the unit mm Hg. Also, out of tribute we use the unit torr (Torricelli) which is interchangeable with mm Hg Also a common unit used with pressure is the standard atmosphere (atm) 1 atm = 760 mm Hg = 760 torr = 101,325 Pa (N/m2) = 29.92 in Hg = 14.7 lb/in2 (psi) Because Pressure = force/area the SI unit of measure is N/m2 This number is called a Pascal (Pa) Sample Exercise The pressure of a gas is measured as 49 torr. Represent this pressure in both atmospheres and then in Pascals The Gas Laws of Boyle, Charles and Avogadro These three laws are used to comprise the Ideal Gas Law These components were found first and later combined as we will see later to form: PV = nRT Boyle’s Law Robert Boyle (25 January 1627 – 31 December 1691) This law relates pressure to volume for a given amount of gas The mathematical way to represent this is PV = k Where P = pressure, V = volume and k = constant Chemistry 121 Notes 16 – Gases Page 2 of 7 This can be rearranged as follows: P ~ 1/V or V ~ 1/P This relationship works for a given sample of gas a given temperature Pressure and volume are inversely proportional Current (very precise) measurement suggest that this relationship still holds true for very low pressures A gas that obeys Boyle’s Law is called an Ideal Gas Sample Problem Sulfur dioxide (SO2), a gas that plays a central role in the formation of acid rain, is found in the exhaust of automobiles and power plants. Consider a 1.53 L sample of gaseous SO2 at a pressure of 5.6 x 103 Pa. If the pressure is changed to 1.5 x 104 Pa, at a constant temperature, what will be the new volume of the gas? Charles’s Law Jacques Alexandre César Charles (12 November 1746 – 7 April 1823) He found a relation between volume and temperature at constant pressure The volume of gas is directly proportional to the temperature The mathematical way to represent this is: V = bT Where, V = volume, b = proportionality constant and T = temperature Temperature is always expressed in Kelvin Chemistry 121 Notes 16 – Gases Page 3 of 7 Sample Problem A sample of gas at 15 0C and 1 atm has a volume of 2.58 L. What volume will this gas occupy at 38 0C and 1 atm? Avogadro’s Law Lorenzo Romano Amedeo Carlo Avogadro (9 August 1776 – 9 July 1856) Avogadro in the early 1800’s found that equal volumes of gases at the same temperature and pressure contain the same number of particles The mathematical way to represent this is: V = an Where, V = volume, n = number of moles and a = proportionality constant For a given gas at a constant temperature and pressure the volume is directly proportional to the number of moles of gas. Sample Problem Suppose we have a 12.2 L sample containing 0.50 mol of oxygen gas (O2) at a pressure of 1 atm and a temperature of 25 0C. If all of this O2 were converted to ozone (O3) at the same temperature and pressure, what would be the volume of the ozone? Chemistry 121 Notes 16 – Gases Page 4 of 7 The Ideal Gas Law We now know the three laws that are used to make the ideal gas law: Boyle’s Law V = k/P Charles’ Law V = bT Avogadro’s Law V = an When combined they form the ideal gas law: P = R(Tn/V) R is the universal gas constant R = 0.08206 L.atm K.mol Rearranging the previous equation for an ideal gas we get: PV = nRT Where P = pressure, V = volume, n = number of moles, R = the universal gas constant and T = temperature (always in Kelvin) The ideal gas law is an equation of state for a gas The state of a gas is its condition at that time The state of a gas is described by its pressure, volume, temperature and number of moles. If three are known the fourth can be determined from the ideal gas law The ideal gas law applies most accurately for pressures < 1 atm and/or high temperatures This law is based on observations (empirical). There is no such thing as an ideal gas but some gases act very close to this limit Chemistry 121 Notes 16 – Gases Page 5 of 7 For our purposes we will use the ideal gas relation for calculations Sample Problem A sample of hydrogen gas (H2) has a volume of 8.56 L at a temperature of 0 0C and a pressure of 1.5 atm. Calculate the moles of H2 molecules present in this gas sample. Sample Problem 2 A sample of methane gas that has a volume of 3.8 L at 5 0C is heated to 86 0C at constant pressure. Calculate its new volume. Chemistry 121 Notes 16 – Gases Page 6 of 7 Sample Problem 3 A sample of diborane gas (B2H6), a substance that bursts into flames when exposed to air, has a pressure of 345 torr at a temperature of -15 0C and a volume of 3.48 L. If conditions are changed so that the temperature is 36 0C and the pressure is 468 torr, what will be the volume of the sample? Sample Problem 4 A sample containing 0.35 mol argon gas at a temperature of 13 0C and a pressure of 568 torr is heated to 56 0C and a pressure of 897 torr. Calculate the change in volume that occurs. Chemistry 121 Notes 16 – Gases Page 7 of 7