Physical Sciences Paper 2 Exam Prep: Organic Molecules & More
advertisement

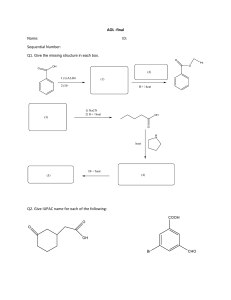
THE LAST PUSH PHYSICAL SCIENCES PAPER 2 PREPARATORY EXAMINATION 2016 COMPILED BY: COLLEN “COACH” MKHOMAZI TABLE OF CONTENTS TOPIC PAGE 1 ACKNOWLEDGEMENTS 3 2 ORGANIC MOLECULES 1 4 – 9 3 ORGANIC MOLECULES 2 10 – 15 4 ORGANIC MOLECULES 3 16 – 21 5 22 – 30 6 MECHANISM & RATES OF REACTIONS CHEMICAL EQUILIBRIUM 7 ACIDS AND BASES 36 – 40 8 ELECTROCHEMICAL REACTIONS 1 41 – 45 9 ELECTROCHEMICAL REACTIONS 2 46 – 50 10 CHEMICAL (FERTILIZER) INDUSTRY 51 – 54 31- 35 2 ACKNOWLEDGEMENTS The author would like to thank the following people for their advice and support in the development of this module: Kehumile Rachel Taunyane Admin Assistant Kutlwanong Centre for Maths, Science and Technology & Lindiwe Nkutha Admin Assistant Kutlwanong Centre for Maths, Science and Technology 3 QUESTION 2 (EASTERN CAPE) ORGANIC MOLECULES 1 The letters below A to F in the table below represent six organic compounds. A B C C3H7C D Propanoic acid E Polyethene F CnH2nO2 Use the information in the table to answer the questions that follow; 2.1 Write down the letter of the compound that … 2.1.1 has a carboxyl group. 2.1.2 is used to make plastic. 2.2 Write down the … 2.2.1 IUPAC name of compound B. 2.2.2 STRUCTURAL FORMULA of the monomer of compound E. 2.3 Compound A is an alkane. Write down the … 2.3.1 GENERAL FORMULA for alkanes. 2.3.2 MOLECULAR FORMALAE of each of the two products obtained during the complete combustion of compound A. 2.4 Compound C is a primary haloalkane: 2.4.1 Write down the STRUCTURAL FORMULA and IUPAC name of a STRUCTURAL ISOMER of compound C. 2.4.2 Classify the isomer in QUESTION 2.4.1 as CHAIN, POSITIONAL or FUNCTIONAL. 2.5 Chemical analysis of compound F shows that it has the following percentage composition: X% carbon (C), Y% hydrogen (H) and 1, 25% oxygen (O). Use a calculation to determine the value of X. 4 QUESTION 2 (WESTERN CAPE) ORGANIC MOLECULES 1 2.1 Consider the organic compounds represented by the letters A to D below. 2.1.1 Explain why compound A is a saturated compound. 2.1.2 Write down the IUPAC name of compound A 2.1.3 Name the name of homologous series that compound B belongs to 2.1.4 Write down the IUPAC name of compound C. 2.1.5 Write down the structural formula for a functional isomer of compound D. 2.1.6 Write down the IUPAC name of the alcohol needed to prepare compound D. 2.2 The ester contains 9, 81 % hydrogen (H), 58, 82% carbon (C) and 31, 37% oxygen (O). The molar mass of the ester is 157 g∙mol-1. 2.2.1 Calculate the molecular formula of the ester. The alcohol used to prepare this ester is ethanol. 2.2.2 What is the name of the other reagent used to prepare the ester? 5 QUESTION 2 (FREE STATE) ORGANIC MOLECULES 1 A C B Butanal H H H C C C C H H O H H C H H E H H D H H H H C H H C H C H C H CH3CH2CCCH3 F H Ethyl ethanoate H Use the information in the table to answer the questions that follow. 2.1 Write down the letter(s) representing: 2.1.1 2.1.2 2.1.3 2.1.4 A compound with the general formula CnH2n+2 A ketone An aldehyde An unsaturated hydrocarbon 2.2 For compound F, write down the: 2.2.1 Structure of the functional group to which it belongs 2.2.2 IUPAC name of the acid and an alcohol needed to prepare F 2.3 Write down the IUPAC name of: 2.3.1 Compound C 2.3.2 Compound D 2.4 Write down the condensed structural formula for C. 2.5 Write down the structural formula of: 2.5.1 A chain isomer of compound E 2.5.2 Compound F 6 QUESTION 2 (GAUTENG) ORGANIC MOLECULES 1 2.1 Write down the name of the homologous series to which each of the following compounds belongs. 2.1.1 CH3CHO 2.1.2 CH3COCH3 2.2.1 Define the term positional isomer. 2.2.2 Write down the structural formula of an isomer of 1-chloropropane. 2.3 The compound responsible for a fruity scent, pentyl butanoate, is prepared in the laboratory. Write down the: 2.3.1 IUPAC names of TWO compounds needed for this preparation 2.3.2 Type of reaction that takes place 2.4 Consider the structural formula of a polymer shown below. Write down the: 2.4.1 IUPAC name of the polymer 2.4.2 Structural formula of the monomer used to prepare this polymer 2.4.3 Type of polymer of which it is an example 7 QUESTION 2 (KZN- 2015) ORGANIC MOLECULES 1 The letters A to G in the table below represent seven organic compounds. H A H C B H O H C C C H H H H H 2-methylpropan-2-ol D H O C C H H O H CH3CH2CH2CHO F E H H C H H C C C H CH3 CH3 H H H C C C Cℓ Br H Use the information in the table (where applicable) to answer the questions that follow. 2.1 Write down the LETTER that represents a compound that: (A compound may be used more than once.) 2.1.1 2.1.2 2.1.3 2.2 Write down the IUPAC name of compound: 2.2.1 2.2.2 2.3 Is an aldehyde. Is a tertiary alcohol. contains a carboxyl group. E F Write down the structural formula of: 2.3.1 2.3.2 a functional isomer of compound B. the functional group of compound A. 8 H 2.4 Write down the letters of two compounds that belong to the same homologous series. 2.5 Write down the general formula for compound E. 2.6 A mixture of compound B, propan-1-ol and concentrated sulphuric acid are together heated in a test tube to produce an organic compound G and water. 2.6.1 Give a reason why the above mixture must not be heated over an open flame. 2.6.2 Write down the name of the type of reaction that occurs. 2.6.3 Write down the IUPAC name for compound G. 2.6.4 Write down the structural formula for compound G. 9 QUESTION 3 (WESTERN CAPE) ORGANIC MOLECULES 2 The relationship between the strength of intermolecular forces and boiling point is investigated using four organic compounds. The compounds and their boiling points are given in the table below. Compound Boiling Point -10 C 36,10 C 27,70 C 820 C Butane Pentane 2-methylbutane 2-methylpropan-2-ol 3.1 Write down the definition of the term “boiling point.” 3.2 Which substance(s) will be liquid at 500 C? 3.3 Name the type of intermolecular forces that is found between butane molecules. 3.4 Refer to the strength of intermolecular forces, the type of intermolecular forces and/or structure of the molecules and energy and explain the difference between the boiling points of the following substances: 3.4.1 pentane and 2-methylbutane 3.4.2 pentane and 2-methylpropan-2-ol 3.5 Which substance will have the lowest vapor pressure at 500 C? 3.6 If 26 butane (C4H10) burns in an excess of oxygen, 34 g CO2 forms. The balance equation for this reaction is given below: 2C4H10 + 13O2→ 8CO2 + 10H2O Calculate the percentage purity of the butane gas. 10 QUESTION 3 (FREE STATE) ORGANIC MOLECULES 2 During a practical investigation the boiling points of five (5) organic compounds, with known molar masses, were determined and the results were recorded in the table below. The five organic compounds are represented by the letter A to E. COMPOUND 3.1 MOLAR MASS (g/mol-1) 44 BOILING POINT (°C) - 42 A CH3CH2CH3 B CH3CH2CH2CH3 58 -1 C CH3CH2CH2CH2CH3 72 36 D CH3CH(CH3)CH2CH3 72 28 E CH3C(CH3)2CH3 72 10 Consider only compounds A, B and C and write down: 3.1.1 The independent variable 3.1.2 The dependent variable 3.1.3 An investigative question 3.2 What is the trend in boiling point from compound C to compound E? Write down INCREASES or DECREASES. Fully explain this trend. 3.3 Consider compound E. 3.3.1 Write down the NAME of the homologous series to which it belongs. 3.3.2 Write down the IUPAC name of this compound. 11 QUESTION 3 (EASTERN CAPE) ORGANIC MOLECULES 2 There are three chain isomers having the molecular formula, C5H12. In a particular investigation, vapour pressure data for the three chain isomers A, B and C is collected and plotted on a graph as shown below. 3.1 Define the term chain isomer. 3.2 Use the graph to estimate the vapour pressure of the straight chain isomer of C5H12at 200 C. 3.3 Write down the STRUCTURAL FORMULA OF compound C. 3.4 Explain the difference in the vapour pressure of compound A and B. In your explanation refer to the STRUCTURE of the molecules, the TYPE and STRENGTH of the INTERMOLECULAR FORCE(S). 12 3.5 The leaners also collected boiling point data for compounds D, E and F as shown in the table below. Compound D E F Condensed Structural Formula CH3OH CH3CH2CH2OH CH3Cℓ2 Boiling point (°C) 78 97 39,6 3.5.1 Write down the NAME of the type of intermolecular force that is responsible for the difference in the boiling points of compound D and E. 3.5.2 Explain the difference in the boiling points of compounds D and F by referring to the TYPE and STRENGTH of intermolecular forces. Compound F is prepared at standard conditions (STP) by the reaction between methane and chlorine as shown by the equation: CH4 (g) + Cℓ2 (g) → CH2Cℓ2 (g) + H2 (g) 3.5.3 Write down the NAME of the type of reaction that leads to the formation of compound F. 3.5.4 In the reaction 21, 88 dm3 of CH4 produces 0,043 kg OF CH2C 2. Calculate the percentage yield in this reaction. 13 QUESTION 3 (GAUTENG) ORGANIC MOLECULES 2 The table below shows data collected for four organic compounds during two practical investigations. The compounds are represented by the letters A, B, C and D. The boiling point of compound B is unknown and recorded as X. Investigation I II 3.1 Organic Compound A B C D CH3CH2CH2CH2CH3 CH3CH(CH2)CH2CH3 CH3CH2CH2CH2OH CH3CH2COOH Relative molecular mass 72,15 72,15 74,12 74,08 Boiling point (0C) 36,1 X 117 141,2 For investigation II, write down the: 3.1.1 Dependent variable 3.1.2 Independent variable 3.1.3 Controlled variable 3.2 Will the boiling point of compound B, be HIGHER THAN, LOWER THAN or EQUAL TO the boiling point of compound A? 3.3 Fully explain the answer to Question 4.2. 3.4 How will the vapor pressure of compound C compare to that of compound D? Choose from HIGHER THAN, LOWER THAN or EQUAL TO. 3.5 Fully explain the answer in Question 4.4 3.6 Write down the STRUCTURAL FORMULA and IUPAC NAME of a FUNCTIONAL isomer of compound D. 14 QUESTION 3 (MPUMALANGA - 2015) ORGANIC MOLECULES 2 A learner investigates the relationship between the structural isomers of pentane and their boiling points. The results obtained were recorded as shown below: COMPOUND Pentane 2-methylbutane 2,2-dimethylpropane MOLECULAR FORMULA C5H12 C5H12 C5H12 BOILING POINT (0C) 36 28 10 3.1 Define the term structural isomer. 3.2 Name the homologous series to which the compounds belong. 3.3 For this investigation write down the: 3.3.1 3.3.2 3.3.3 Dependent variable Independent variable Conclusion that can be drawn from the above results 3.4 Refer to MOLECULAR STRUCTURE, INTERMOLECULAR FORCES and ENERGY needed, to explain your conclusion in QUESTION 3.3.3. 3.5 What precaution should the learners take when carrying out the experiment? Give reason. 15 QUESTION 4 (WESTERN CAPE) ORGANIC MOLECULES 3 The flow diagram below shows different organic reactions. A and B represent different organic compounds. 4.1 Write down the name of the type of elimination reaction that occurs in reaction 1. 4.2 During Reaction 2, CH2 = CH2 undergoes polymerization to form compound A. For this reaction, write down the: 4.2.1 the type of polymerization 4.2.2 NAME of compound A 4.2.3 condensed structure of compound A. 4.3 Use structural formulas to write a balanced chemical equation for the reaction that occurs during reaction 3. 4.4 What is the name for the type of addition reaction that is represented by reaction 3? 4.5 Write down the IUPAC name for compound B. 4.6 Write down TWO reaction conditions that will favor reaction 4. 4.7 Consider reaction 5 4.7.1 Name the type of reaction that occurs. 4.7.2 Name the most favorable reaction conditions for this reaction. 4.7.3 Except for bromo-ethane, give the name of another product that forms during this reaction. 16 QUESTION 4 (FREE STATE) ORGANIC MOLECULES 3 The flow diagram below shows how alcohols can react to form other organic compounds. Compound X CH3CHCℓCH3 Secondary Alcohol Reaction A +Concentrated KOH Reaction C Reaction B Alkene Reaction D +H2 25 Alkene 4.1 Write down the type of reaction represented by reaction: 4.1.1 A 4.1.2 B 4.1.3 D 4.2 In reaction B, compound X is converted to an alkene. Write down the: 4.2.1 IUPAC name of compound X 4.2.2 Balanced equation for the reaction B, using structural formulae 4.3 Reaction C takes place in the presence of a strong acid. 4.3.1 Explain the term secondary alcohol 4.3.2 Write down the IUPAC name of the alcohol used 17 QUESTION 4 (GAUTENG) ORGANIC MOLECULES 3 4.1 Consider the structural formula of an organic compound below. 4.1.1 Is the above compound a SATURATED or an UNSATURATED hydrocarbon? Give a reason for the answer. 4.1.2 Write down the IUPAC name of the above compound. 4.2 Draw the structural formula of 4, 4-dimethylhexan-2-one 4.3 In industry, ethene is used to synthesise a variety of organic compounds. The flow diagram below illustrates some of the reactions that ethene can undergo. Write down: 4.3.1 The general formula of the homologous series to which ethene belongs. 4.3.2 A balanced equation, using structural formulae, for reaction B. 4.3.3 The type of reaction represented by G. 18 4.3.4 The FORMULA of the inorganic reactant needed for reaction F. 4.3.5 The letter that represents a dehydrohalogenation reaction 4.3.6 The difference between the base used in reaction E and the base used in reaction G. 4.3.7 The NAME or FORMULA of the catalyst used in reaction C. 19 QUESTION 4 (EASTERN CAPE) ORGANIC MOLECULES 3 4.1 The flow diagram below shows two organic reactions in which 2-chlorobutane reaches with potassium hydroxide (KOH) under different reactions conditions. 2-chlorobutane KOH Reaction2 Alkene (Major Product) Reaction 1 KOH Alcohol Use the information in the flow diagram to answer the questions that follow: 4.1.1 Write down the …. (a) type of reaction of which Reaction1 is an example. (b) IUPAC name of the alcohol. 4.1.2 Which ONE of the reactions, 1 or 2, uses concentrated potassium hydroxide? 4.1.3 Write down the STRUCTURAL FORMULA of the alkene. 4.2 A small sample of propyl methanoate is prepared in a school laboratory using an alcohol and a carboxylic acid in the presence of a catalyst. The reaction mixture is heated in a water bath. Write down the … 4.2.1 reason why the reaction mixture is heated in a water bath instead of heating directly. 4.2.2 STRUCTURAL FORMULA of the alcohol. 4.2.3 IUPAC name of the carboxylic acid. 20 QUESTION 4 (KZN – 2015) ORGANIC MOLECULES 3 The flow diagram below shows the reactions of 2-chloro-3-methylpentane under different conditions. Reaction 1 2-chloro-3-methylpentane Reaction 2 Compound A alkene Compound B 4.1 Classify 2-chloro-3-methylpentane as SATURATED or UNSATURATED and give a reason for the answer. 4.2 Reaction 1 takes place in the presence of dilute sodium hydroxide. Name the type of substitution reaction that takes place. 4.3 Write down the: 4.3.1 Structural formula for compound A. 4.3.2 TWO reaction conditions for reaction 2. 4.3.3 Name of the type of reaction of which reaction 2 is an example. 4.3.4 Name of the alkene formed in reaction 2. 4.4 Compound B is formed when 2-chloro-3-methylpentane reacts in the presence of concentrated sodium hydroxide. 4.4.1 Write down another reaction condition required for this reaction. 4.4.2 Classify this reaction as SUBSTITUTION, ADDITION or ELIMINATION. 21 QUESTION 5 (EASTERN CAPE) Learners investigate some of the factors that influence the rate of chemical reaction. In the experiment they add equal amounts of each of three different metals separately to equal volumes of excess dilute hydrochloric acid solution. In each experiment the acid completely covers the metal. The data obtained is recorded as in the table below: Experiment Amount of metal powder 1 2 3 0,1 mol Zn 0,1 mol Mg 0,1 mol Cu Change in temperature of solution (°C) (Tfinal–Tinitial) +23 +37 0 Time taken to run to completion 25,2 8,3 No reaction 5.1 Is the reaction Experiment 1 ENDOTERMIC or EXOTHERMIC? Give a reason for your answer. (Use the information in the table). 5.2 Which factor influencing reaction rate is investigated? 5.3 How will the total volume of hydrogen gas produced in Experiment 2 compare with the total volume of hydrogen gas produced Experiment 1 at the end of the reactions? Write down HIGHER THAN, EQUAL TO or SMALLER THAN. Give a reason for your answer. 22 The graphs obtained for Experiment 1 and Experiment 2, labelled as 1 and 2 respectively, are sketched on the same set of axes as shown below: Volume of hydrogen gas (dm3) 5.4 0 t1 t2 t3 Time(s) 5.4.1 In which experiment does the reaction occur at a higher reaction rate at time t1? 5.4.2 Explain the answer to QUESTION 5.4.1 by referring to the relative strength of reducing agents involved. 5.5 In another experiment, Experiment 4, the same reaction conditions are repeated as in Experiment 2. But the reaction mixture is heated. The rate of reaction is HIGHER for Experiment 4 than 2. Explain why the reaction rate is HIGHER for Experiment 4 than 2 by referring to the collision theory. 23 QUESTION 5 (WESTERN CAPE) MECHANISM & RATES OF REACTIONS A chemist wishes to determine the rate of reaction of zinc with hydrochloric acid. The equation for the reaction is: Zn(s) + HCℓ (aq) → ZnCℓ2 (aq) + H2(g) A piece of zinc is dropped into1 dm3 of 0,1 mol∙dm3 HCℓ and the following data were Time (s) Mass of Zinc (g) 0 0,016 4 0,012 8 0,010 12 0,009 16 0,008 20 0,008 5.1 Calculate the average rate of the reaction for the first 12 s in mol∙s-1. 5.2 Explain why the mass of the zinc remained constant after 16 s. 5.3 Explain how the rate of the reaction changes as the time passes. 5.4 A graph that shows the amount of H2 gas that was produced against time for this reaction is shown below: Redraw the graph and indicate on the same set of axis 5.4.1 A second graph, named X that will be obtained if the same volume of HCℓ with a lower concertation was used. 5.4.2 A third graph, named Y that will be obtained if zinc powder of the same mass, instead of zinc granules were used. 24 The Maxwell-Boltzmann distribution curve below represents the number of particles against kinetic energy at 3000 C. 5.5 Redraw this curve in the ANSWER BOOK. On the same set of axes, sketch the curve that will be obtained at 4000 C. Clearly label the curves as 3000 C and 4000 C respectively. 25 QUESTION 5 (FREE STATE) MECHANISM & RATES OF REACTIONS In an investigation of the rate of reaction, excess magnesium powder is added to dilute hydrochloric acid at room temperature. The following spontaneous reaction takes place: Mg(s) + 2HCℓ(aq) → MgCℓ2(aq) + H2(g) ∆H < 0 5.1 Define the term spontaneous reaction. 5.2 Write down the limiting reagent for the above reaction. 5.3 How will each of the following changes affect the rate of the reaction between magnesium and hydrochloric acid according to the above reaction? Choose from INCREASES, DECREASE or REMAINS THE SAME. 5.3.1 The same mass of magnesium ribbon is used instead of powder. 5.3.2 A more concentrated solution of hydrochloric acid is used. 5.3.3 The diluted hydrochloric acid solution is heated before being added to the magnesium. 5.4 Use the collision theory to explain your answer in QUESTION 5.3.3. 26 QUESTION 5 (GAUTENG) MECHANISM & RATES OF REACTIONS A group of learners use the reaction between magnesium and nitric acid to investigate ONE of the factors that affects reaction rate. The reaction rate that takes place is represented by the balanced equation below Mg(s) + 2HNO3(aq) → Mg(NO3)2(aq) + H2(g) They add magnesium ribbon to dilute nitric acid and measure the mass of magnesium used per unit time. The experiment is repeated using concentrated nitric acid. 5.1 Write down an investigative question to this investigation The results obtained for the reaction with dilute nitric acid are represented in the graph below. 5.2 Which substance Mg OR HNO3, is in excess? Use the information from the graph to give a reason for your answer. 5.3 Define the term reaction rate. 5.4 Calculate the average rate of the reaction (in gram per second) during the first 30 s. 5.5 Copy the above graph in your answer book. On the same set of axes use a DOTTED LINE to show the curve that will be obtained when concentrated nitric acid is used. NO numerical values are required. 27 QUESTION 5 (EASTERN CAPE – 2015) MECHANISM & RATES OF REACTIONS Learners use the reaction of a sodium thiosulphate solution (Na2S2O3) with a hydrochloric acid solution (HCℓ) to investigate the factors which influence reaction rate. The balanced equation for the reaction is: Na2S2O3 (aq) + 2HCℓ (aq) → H2O (ℓ) + SO2 (g) + 2NaCℓ (aq) + S (s) The time lapse from the moment of mixing equal volumes of the two solutions until a certain degree of turbidity (sulphur precipitation formation) appeared, is taken as a measure of the rate of the reaction. 5.1 Consider INVESTIGATION A: Experiment 1 Experiment 2 Experiment 3 Temperature (°C) Concentration of Na2S2O3 (mol·dm-3) Concentration of HCℓ (mol·dm-3) Time (s) 20 20 20 0,5 0,9 1,4 0,5 0,5 0,5 40 25 15 5.1.1 For investigation A, name the: (a) (b) Dependent variable Independent variable 5.1.2 What conclusion can be drawn from the results of investigation A? 5.1.3 Which ONE of the two reactants (Na2S2O3 or HCℓ) in experiment 1 of investigation A? 5.2 Consider INVESTIGATION B: Experiment 4 Experiment 5 Experiment 6 Temperature (°C) Concentration of Na2S2O3 (mol·dm-3) Concentration of HCℓ (mol·dm-3) Time (s) 20 30 50 0,5 0,5 0,5 0,5 0,5 0,5 40 20 10 5.2.1 In which experiment is the rate of the reaction the fastest? Give a reason for your answer. 5.2.2 Explain your observation in QUESTION 5.2.1 in terms of the collision theory. 28 QUESTION 5 (GAUTENG) MECHANISM & RATES OF REACTIONS 5.1 Curve L shown below, is the Maxwell-Boltzman distribution curve for a gas in a closed container at 2500C. 5.1.1 Name the energy represented by N. 5.1.2 Write down the change in reaction conditions that resulted in: (a) Curve M (b) Curve K Use the collision theory to explain how this change affects the rate of the reaction. 5.2 A series of experiments is conducted to compare the reactions of zinc foil, zinc powder, copper powder and a mixture of zinc powder and copper pieces with dilute sulfuric acid of concentration 1 mol∙dm-3. Hydrogen gas is produced in all test tubes where a reaction takes place. The diagram shows the test tubes, some time after the metals have been added to the acid. 29 5.2.1 Refer to the relative strengths of oxidizing agents or reducing agents to explain why no reaction takes place in test tube C. 5.2.2 How does the rate of the reaction in test tube B compare to that in (Choose from GREATER THAN, SMALLER THAN or EQUAL TO.) (a) test tube A? Give a reason for your answer. (b) test tube D? Give a reason for your answer. 30 QUESTION 6 (GAUTENG) CHEMICAL EQUILIBRIUM The reaction represented below reaches equilibrium in a closed container. TEMPERATURE EQUILIBRIUM CONSTANT (KC) 400 1,58 X 10-3 600 1,58 X 10-9 The equilibrium constants for this reaction at two different temperatures are given in the table below 6.1.1 Is the forward reaction ENDOTHERMIC or EXORTHERMIC? 6.1.2 Use Le Chatelier’s principle to explain your answer to Question 7.1.1. 6.1.3 The pressure in the container is now decreased by increasing the volume of the container. What effect will this have on the value of the equilibrium constant? 6.2 Exactly 24,0 mol SO3 (g) is sealed in an empty 2,0 dm3 container. The reaction reaches equilibrium at 700K after 8 minutes, according to the following balanced equation 2SO3(g) ↔ 2SO2SO2(g) + O2(g) If the reaction mixture contains 10,0 mol O2(g) at equilibrium, calculate the equilibrium constant (Kc) at 700 K. 31 QUESTION 6 (EASTERN CAPE) CHEMICAL EQUILIBRIUM The reaction represented by the equation below reaches equilibrium in a closed container. N2(g) + 3H2(g) ⇋ 2NH3(g) 6.1 ΔH = -92 kJ Is the above equilibrium HOMGENEOUS or HETERGENEOUS? Give a reason for your answer. 6.2 Changes were made to the TEMPERATURE, PRESSURE and CONCENTRATION of the above equilibrium mixture. The graphs below represent the results obtained. 6.2.1 What changes were made to the reaction conditions at each of the following times? (a) t1 (b) t2 6.2.2 6.3 How does the rate of the forward reaction compare to the rate of the reverse reaction between0 and t1? Write down HIGHER THAN, LOWER THAN or EQUAL TO. Equal number of moles of hydrogen gas and nitrogen gas are injected into a sealed 1 dm3 container. When the reaction reaches equilibrium at temperatureT1 it is found that 10% of the original amount of hydrogen is left in the container. The value of Kc at temperature T1 is 1,426 × 103. 6.3.1 Calculate the initial mass of N2 in the container. 6.3.2 Use your knowledge of Le Chatelier’s principle to explain how anincrease in temperature will affect the value of Kc. 32 QUESTION 6 (WESTERN CAPE) CHEMICAL EQUILIBRIUM 33, 6 g N2 and 24 g H2 are placed in a 5 dm3 sealed container and react according to the following balanced equation: N2(g) + 3 H2(g) ⇌ 2NH3(g) 6.1 Explain the term “dynamic chemical equilibrium” 6.2 Give a reason why this reaction will only reach equilibrium in a SEALED container. At 573 K the equilibrium constant for this reaction is 4,34 x 10-3. When this equilibrium is reached, there is 5,6 g N2 gas left inside the container. 6.3 Calculate the concentration of both reactants when the reaction reached equilibrium for the first term. 6.4 Calculate the concentration of NH3(g) that forms when this reaction reached equilibrium. 6.5 How would it influence the yield of NH3 if a smaller container than the 5 dm3 is used? Write only INCREASES, DECREASES or REMAINS THE SAME. The temperature of this reaction increases from 573 K to 700 K. At this new temperature the equilibrium constant for this reaction becomes 1, 04 x 10-4. 6.6 Is this an example of an endothermic or exothermic reaction? 6.7 Explain the answer to QUESTION 6.6 33 QUESTION 6 (FREE STATE) CHEMICAL EQUILIBRIUM During the industrial preparation of ammonia, nitrogen gas and hydrogen gas react in a closed container until the following equilibrium is established at a constant temperature of 472 °C. Kc is 0, 1 at this temperature of 472 °C. The volume of the container is 0, 5 dm3. N2 (g) + 3H2 (g) 2NH3 (g) ∆H = - 92kJ∙mol-1 The equilibrium concentrations are: [NH3] = 2, 7 x 10-3 mol∙dm-3 [H2] = 1,221 x 10-1 mol∙dm-3 6.1 Write down the term for the underlined phrase. 6.2 Write down the NAME or FORMULA of the catalyst used in this reaction. 6.3 After equilibrium has been established, the temperature remained constant. Explain this observation. 6.4 Calculate the initial mass of nitrogen gas. 6.5 Explain why such a high temperature is used although the yield is low. 34 QUESTION 6 (KZN – 2015) CHEMICAL EQUILIBRIUM The following equation represents a hypothetical reaction that reaches equilibrium in a 2 dm3 closed container at 500 °C after 8 minutes. 2AB3(g) ⇌ 2AB2(g) + B2(g) ΔH < 0 Graph of amount of gas versus time 10 Amount of gas (mol) 8 AB2(g) 6 AB3(g) 4 B2(g) 2 0 0 4 8 12 16 Time (minutes) 20 24 28 6.1 At 16 minutes, one of the conditions affecting the equilibrium is changed at constant volume and a new equilibrium is thereafter established. Calculate the Kc value at the new equilibrium. 6.2 Which condition, CONCENTRATION or TEMPERATURE was changed? 6.3 Was the condition identified in QUESTION 6.2 INCREASED or DECREASED? 6.4 Use Le Chatelier's principle to explain the answer to QUESTION 6.3. 6.5 How does the equilibrium constant, Kc, between t = 8 minutes and t = 16 minutes compare to that between t = 24 minutes and t = 32 minutes? Write down only GREATER THAN, SMALLER THAN or EQUAL TO. 6.6 How will the Kc value be affected if the volume of the container, is decreased from 2 dm3 to 1 dm3 after 32 minutes, while keeping the temperature constant. 35 32 QUESTION 7 (EASTERN CAPE) ACIDS AND BASES Learners perform a titration to standardise a dilute sodium hydroxide (NaOH) solution. They use standard oxalic acid (H2C2O4.2H2O) solution of concentration 0, 02 mol∙dm-3. The titration is repeated three times after which the average volume readings are calculated as shown in the table below: Titrations 1 2 3 Average Volume of oxalic acid solution (cm3) 25 25 25 25 7.1 What does the term standard solution mean? 7.2 Give a reason why the titration is repeated three times. 7.3 The balanced equation for the reaction taking place is: Volume NaOH solution (cm3) 20,24 19,80 19,87 19,97 H2C2O4.2H2O(aq) + 2NaOH(aq) → Na2C2O4(aq) + 4H2O(ℓ) Calculate the number of moles of oxalic acid reacting. 7.4 The dilute solution of sodium hydroxide used in the titration was obtained by adding 90 cm3 of water to 10 cm3 of a sodium hydroxide solution. Calculate the pH of the sodium hydroxide solution BEFORE dilution. 36 QUESTION 7 (WESTERN CAPE) ACIDS AND BASES 27 g Ba (OH)2 is dissolved in 0,2 dm3 water at 250 C. A drop of bromothymol blue is added to the solution and it turns blue. 7.1 Explain why Ba (OH)2 is a strong base. 7.2 Calculate the pH of Ba (OH)2 –solution HCℓ with a concentration of 2,5 mol∙dm-3 is added to the Ba (OH)2-solution as shown in the sketch below The balanced equation for this reaction is Ba(OH)2 + 2HCℓ →BaCℓ2 + 2H2O 7.3 Write down the name of the apparatus labelled P. 7.4 Write down the Lowry-Brønsted definition of an acid. 7.5 Calculate the minimum volume of HCℓ that must be added to the reaction so that it changes colour to yellow. 7.6 Explain why bromothymol blue is a better choice than phenolphthalein to indicate the neutralization in the reaction above. 7.7 What would happen to the colour of the solution if 200 cm3 water is added after the HCℓ was added and the solution turned yellow? Write only REMAINS YELLOW or CHANGES TO BLUE. 37 QUESTION 7 (FREE STATE) ACIDS AND BASES 7.1 Hydrochloric acid is a highly corrosive strong acid with many industrial uses. When 0, 02 dm3 of sodium hydroxide is added to 0, 15 dm3 of diluted hydrochloric acid of concentration 0, 03 mol.dm-3, the pH of the mixture changes to 4. 7.1.1 Give a reason why hydrochloric acid is classified as a strong acid. 7.1.2 Write down a balanced chemical equation to show the dissociation of HCℓ in water. 7.1.3 Will the final mixture be acidic or basic? Give a reason for your answer by referring to the pH of the mixture. 7.1.4 Calculate the final concentration of the H+ ion in the mixture. 7.1.5 Calculate the original concentration of the sodium hydroxide solution. 7.2 Calcium carbonate solutions provide living organisms with the substance they need to grow their protective shells and skeletons. For example, eggshells are composed of calcium carbonate. Grade 12 learners decide to calculate the percentage calcium carbonate in eggshells at STP. They take 5 g of crushed eggshells and react it with excess hydrochloric acid according to the following equation: CaCO3(s)+ 2 HCℓ(aq) → CaCℓ2(aq) + CO2(g) + H2O(ℓ) The carbon dioxide gas produced is collected and found to be 1,06 dm3 after all the calcium carbonate has reacted. Calculate the percentage calcium carbonate in 5 g eggshells. Show all the calculations 38 QUESTION 7 (GAUTENG) ACIDS AND BASES 7.1 A factory accidentally spills sulpuric acid into a nearby river. The fish species in the river CANNOT survive in water with a pH LOWER THAN 5,8. Analysis of water samples from the river shows that the hydrogen ion concentration is 5,6 x 10-6 mol.dm-3 Show with the aid of a calculation that the fish will not survive in the river. 7.2 A sample of seawater is treated with 500 cm3 of a 2,5 mol.dm-3 sodium hydroxide solution to remove the magnesium ions. The reaction that takes place is represented by the following balanced equation: Mg(NO3)2(aq) + 2NaOH(aq) → Mg(OH)2 (s) + 2NaNO3(aq) After removal of the precipitate, the excess NaOH(aq) is titrated with 95 cm3 of a 0,2 mol.dm3 sulphuric acid solution. The balanced equation for the reaction is: 2NaOH(aq) + H2SO4(aq) → Na2SO4(aq) + 2H2O(ℓ) Calculate: 7.2.1 The number of moles sodium hydroxide added to the seawater 7.2.2 The original mass of magnesium nitrate in the seawater 39 QUESTION 7 (MPUMALANGA- 2015) ACIDS AND BASES 7.1 A bottle in a laboratory contains dilute sulphuric acid of unknown concentration. Learners wish to determine the concentration of the sulphuric acid solution. To do this they titrate the sulphuric acid against a standard potassium hydroxide solution. The balanced equation for the reaction taking place is: 2KOH + H2SO4 → K2SO4 → 2H2O 7.1.1 What is the standard solution? 7.1.2 Calculate the mass of KOH which he must use to make 300 cm3 of a 0.2 mol∙dm-3 KOH solution. 7.1.3 Calculate the pH of the 0.2 mol∙dm-3 KOH solution. 7.1.4 Which one of the indicators listed in the table below should he use in this titration? Explain your answer. INDICATOR pH Methyl orange 2.9 – 4.0 Methyl red 4.4 – 6.0 Bromothymol blue 6.0 – 10.0 Phenolphthalein 8.3 – 10.0 7.1.5 During the titration the learners finds that 15 cm3 of the KOH solution neutralises 20 cm3 Of the H2SO4 solution. Calculate the concentration of the H2SO4 solution. 7.2 An impure sample of calcium oxalate, CaC2O4, with a mass of 0.803 g, is titrated with 15.70 cm3 of a 0.101 mol∙dm-3 KMnO4. The net reaction is…. 2MnO4 --+ 5C2O42- + 16H+ → 2Mn2+ 10CO2 + 8H2O Calculate the percentage purity of the CaC2O4 in the original sample. 40 QUESTION 8 (EASTERN CAPE) ELECTROCHEMICAL REACTIONS 1 8.1 Consider the electrochemical cell below. Electrode Y in half cell B is an inert metal 8.1.1 8.1.2 8.1.3 8.1.4 8.1.5 For the electrochemical cell, write down … ONE function of the saltbridge. the voltmeter reading when the cell reaction reaches equilibrium. name or formula of the inert metal Y. name or formula of the oxidising agent. half reaction taking place on the surface of electrode Y. 8.2 The initial EMF of the above electrochemical cell under standard conditions is0,83 V. Identify metal X by calculation. 8.3 Write down the cell notation for this cell. 41 QUESTION 8 (WESTERN CAPE) ELECTROCHEMICAL REACTIONS 1 A galvanic cell contains a platinum and an aluminium electrode. The platinum electrode serves as an inert electrode for the cobalt ion half reaction. The half reactions for the redox reaction that occur in this cell are given on the table of standard reduction potentials as follows: HALF REACTION EƟ (V) Al 3+ + 3e-⇌ Al -1,66 Co3+ + e-⇌ Co2+ +1,81 8.1 At which electrode are electrons released to the external circuit? Write only Pt or Al. 8.2 Write down the 8.2.1 Oxidation reaction that occurs in this cell. 8.2.2 The name or formula of the reducing agent in this reaction. 8.3 How does the mass of the aluminium electrode change during this reaction? Write only INCREASES or DECREASES. 8.4 Write down the cell notation for this reaction. The aluminium electrode is replaced by a zinc electrode 8.5 Calculate the new initial cell potential of this cell when it is in operation under standard conditions. 42 QUESTION 8 (FREE STATE) ELECTROCHEMICAL REACTIONS 1 An electrochemical cell is set up at standard conditions as shown below. The electron flow is as indicated. The reading on the voltmeter is 1,07V. e- V X Ni salt bridge Ni 2+ (aq ) X + (aq ) 8.1 Write down ONE function of the salt bridge. 8.2 Which electrode of the cell is the anode? Write only X or Ni. 8.3 Using a calculation identify the unknown metal X. 8.4 Write down the balanced net (overall) equation for the above cell. Metal X is now replaced by zinc. 8.5 For the new galvanic cell, write down: 8.5.1 The NAME or FORMULA of the oxidising agent 8.5.2 The half reaction for the ANODE 8.5.3 The cell notation of the new cell 43 QUESTION 8 (GAUTENG) ELECTROCHEMICAL REACTIONS 1 An electrochemical cell is set up as shown in the diagram below. 8.1 What is the function of the salt bridge? 8.2 Briefly explain the main difference between the cell illustrated above and an electrolytic cell by referring to the energy that is transferred. 8.3 Which electrode, Fe or Pt, is the cathode? Give a reason for your answer. 8.4 Write down the FORMULA of the reducing agent in this cell. 8.5 Write down the overall cell reaction that takes place in this cell. 8.6 Calculate the initial reading on the voltmeter when this cell functions under standard conditions. 44 QUESTION 8 (MPUMALANGA- 2015) ELECTROCHEMICAL REACTIONS 1 A learner sets up an electrochemical cell as represented in the diagram below. The cell consist of a Mg electrode dipped in a Mg(NO3)2 solution, and a Pb electrode dipped into a Pb(NO3)2 solution. 8.1 8.2 Write down the half-reaction that takes place in half cell A. Write down the overall net reaction for the cell. 8.3 Calculate the emf of this cell. 8.4 How will an increase in [Mg2+(aq)] influence the value of the cell’s emf calculated in QUESTION 8.3. Write down only INCREASES, DECRASES or STAYS THE SAME. 8.5 Consider the following apparatus consisting of galvanic cell joined to an electrolytic cell.. 8.5.1 On the diagram above, in which direction will electrons flow in the top wire as the reaction proceeds? Choose between Pt to Ag OR Ag to Pt. 8.5.2 Which metal is cathode in cell A? For Cell B, write down the equation for the reactions taking place at the: 8.5.3 Anode 8.5.4 Cathode 45 QUESTION 9 (EASTERN CAPE) ELECTROCHEMICAL REACTIONS 2 9.1 Two different cells, A and B are shown in the diagrams below. Cell A contains a concentrated solution of sodium chloride (NaCℓ) and cell B contains a concentrated solution of copper (II) chloride (CuCℓ2). P, Q, R and S are identical carbon electrodes. Chlorine gas is formed at electrode P and S Power source Power source P R Q S Na Cℓ (aq) Cell B Cell A CuCℓ2 (aq 9.1.1 Is cell AELECTROLYTIC or GALVANIC? Give a reason for your answer. 9.1.2 Write down the equation for the half reaction taking place at electrode Q. 9.1.3 Write down the NAME or SYMBOL of the product formed at electrode R. 9.1.4 What happens to the concentration of the electrolyte in cell B when the cell is in operation? Write down INCREASES, DECREASES or REMAINS THE SAME. Give a reason for the answer. 9.2 Aluminium is obtained from alumina (Aℓ2O3) using an electrolytic process. In the process, cryolite (Na3AℓF6), is added to alumina in order to reduce the melting point of alumina. 9.2.1 Give a reason why it is necessary to melt alumina before electrolysis. 9.2.2 The electrolyte in the cell contains Na+ ions. Refer to the attached Table of Standard reduction Potentials to explain why the Na+ ions will not affect the purity of the aluminium obtained during this process. 46 QUESTION 9 (WESTERN CAPE) ELECTROCHEMICAL REACTIONS 2 A solution of sodium chloride in water (NaCℓ + H2O) is used as an electrolyte in an electric cell that is set up as shown in the sketch below: The equation for the nett cell reaction that occurs, is 2NaCℓ (aq) + 2H2O(ℓ) →2NaOH(aq) + H2(g) + Cℓ2(g) 9.1 Define the term “electrolysis”. 9.2 Identify 9.2.1 gas S 9.2.1 gas R 9.3 Explain why sodium metal, Na(s), does not form during the electrolysis of NaCℓ. 9.4 Initially 0,5 dm3 of the NaCℓ electrolyte with a concentration of 2,5 mol∙dm-3 is used. How many moles of the electrolyte will be left after 2,24 dm3 Cℓ2 formed at STP? 47 QUESTION 9 (FREE STATE) ELECTROCHEMICAL REACTIONS 2 Electrolysis is an important industrial process used to decompose compounds, extract metals from their ores and to purify metals like gold or copper. The simplified diagram below represents an electrolytic cell used in the extraction of aluminium. ( -) ( -) (+) (+) A ℓ2O 3 Carbon electrode Na3 AℓF6 (molten cryolite) Molten aluminium 9.1 Write down the energy conversion that takes place in this cell. 9.2 Write down the name of the mineral ore of aluminium. 9.3 Write down the role played by cryolite in this cell. 9.4 The carbon rods need to be replaced from time to time. Write down the balanced chemical equation that explains why they need to be replaced. 9.5 Write down the half-reaction at the cathode. 9.6 Write down TWO negative impacts of this cell on the environment. 9.7 It is preferable to recycle aluminium products rather than extracting it from its mineral ore. Give a reason to substantiate the above statement. 48 QUESTION 9 (GAUTENG) ELECTROCHEMICAL REACTIONS 2 The diagram below shows a simplified cell used to electroplate an iron spoon with silver. 9.1 Define the term electrolyte. 9.2 Write down the: 9.2.1 NAME of substance that can be used as electrolyte in this cell 9.2.2 NAME or FORMULA of the substance which is oxidized 9.2.3 Half-reaction that takes place at the cathode 9.3 Calculate the number of electrons transferred if 2 g of silver is plated on the spoon. 49 QUESTION 9 (EASTERN CAPE -2015) ELECTROCHEMICAL REACTIONS 2 A learner sets up an electrolytic cell, represented in the diagram below, to purify copper which contains platinum and silver impurities. During the purification of 28 g of the impure copper, 0,8 mol of electrons were transferred from the anode to the cathode. 9.1 Calculate the number of copper atoms formed at the cathode. 9.2 The copper used for electrical wiring and cables must be 99, 99% pure. Determine by calculation whether the IMPURE copper sample is suitable for use in electrical wiring and cables. (Assume that all the copper at anode has reacted.) During the purification, a sludge containing the metals platinum and silver forms at the bottom of the container. 9.3 Use the relative strengths of reducing agents to explain why platinum and silver atoms are not oxidised during the purification of copper. 50 QUESTION 10 (EASTERN CAPE) CHEMICAL (FERTILIZER) INDUSTRY The flow diagram below shows the steps involved in the industrial preparation of ammonia and nitric acid. 10.1 Write down the name of … 10.1.1 gasA. 10.1.2 the catalyst used in the preparation of ammonia. 10.2 The reaction in STEP 2, reaches equilibrium according to the equation below: 2NO(g) + O2(g) ⇋ 2NO2(g) ΔH = -149,1 Kj Calculate the amount of the net energy released (in kJ) by the reaction when 1,12 g of the initial amount of NO(g) is used up. 10.3 Nitric acid reacts with ammonia to produce a fertiliser. 10.3.1 Classify the reaction taking place as PROTOLYTIC or REDOX. 10.3.2 Write down a balanced equation for the reaction. 10.4 A 50 kg bag of N: P: Kfertiliser is labelled 7:1:3(60). Calculate the mass of potassium in the bag of fertiliser. 10.5 Name ONE negative effect of the overuse of nitrogen based fertilisers on the environment. 51 QUESTION 10 (WESTERN CAPE) CHEMICAL (FERTILIZER) INDUSTRY 10.1 The diagram below describes the process for making (NH4)2SO4. Study the diagram and answer the questions that follows: 10.1.1 Identify gasA that is used to produces SO3(g). 10.1.2 Give the name of processB that is used for the industrial production of NH3. 10.1.3 Write down the FORMULA for acid C that is used to produce (NH4)2SO4 as shown in the diagram 10.1.4 Give the name of processD that is used to produce acid C 10.2 A certain bag of fertilizer is labelled as shown below: 10.2.1 Name two primary nutrients that is contained in this fertilizer. 10.2.2 Calculate the number of moles of phosphorous present in 40 g fertilizer. 52 QUESTION 10 (FREE STATE) CHEMICAL (FERTILIZER) INDUSTRY Different processes used in the preparation of fertiliser E are represented in the flow diagram below. Oxygen Sulphur Gas A Process X Gas B Haber Process Oleum Compound D Compound C Fertiliser E 10.1 Use the above information and write down the: 10.1.1 NAME or FORMULA of gas A 10.1.2 Name of process X 10.1.3 NAME or FORMULA of the catalyst used in process X 10.1.4 FORMULA of oleum 10.1.5 Balanced equation for the preparation of fertiliser E 10.2 Describe ONE negative impact on humans when fertiliser runs off into dams and rivers as a result of rain. 10.3 Write down the NAME of the most important primary nutrient required to enhance: 10.3.1 Leaf growth of spinach 10.3.2 Flower and fruit production of peach trees 10.4 Which ONE of the three primary nutrients is absorbed by plants the least? 53 QUESTION 10 CHEMICAL (FERTILIZER) INDUSTRY 10.1 A fertilizer bag is labelled 1:4:2(30). Explain the meaning of the: 10.1.1 Ratio, 1:4:2 10.1.2 Value in brackets, (30) 10.2 Consider the two nitrogen containing fertilizer: Ammonium nitrate: Urea: 10.3 NH4NO3 (NH2)2CO The flow diagram below shows two industrial processes, A, and B, used in the production of fertilizers. Write down the: 10.3.1 NAME of process A 10.3.2 balanced equation for the reaction that takes place in the process B 10.3.3 FORMULA and the NAME of the fertilizer represented by Y 54