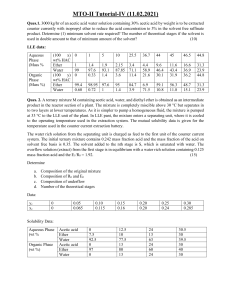
EP325/BEP3043 Separation Process Lesson 7 LIQUID-LIQUID EXTRACTION EXTRACTION •Removal of one or more components (solutes) from solids or liquids using liquid solvent LIQUID-LIQUID EXTRACTION/SOLVENT EXTRACTION •Separation of two miscible liquids using another liquid (solvent) •Eg. Vitamin A and D (solute) from fish oil (inert liquid) using liquid propene (solvent) SOLID-LIQUID EXTRACTION/LEACHING •Separation of solutes from solid using liquid solvent •Eg. Soya milk (solute) from soya bean (inert solid) using water (liquid solvent) LIQUID-LIQUID EXTRACTION • Takes advantage of the relative solubility of solutes in immiscible or nearly immiscible liquids • Solute dissolves more readily in the solvent in which it has a higher solubility • Distribution Coefficient, K, determines the ratio of the concentration of the solute in each liquid. WHY TO CHOOSE LIQUID-LIQUID EXTRACTION METHOD ??? • Separation by distillation is ineffective or difficult • Boiling points of mixtures are close • Flexibility in operation conditions choice is desired • More than two components are present • The material is heat sensitive COUNTER CURRENT EXTRACTION • Two immiscible fluids, usually one light and one heavy fluid, flowing continuously in opposite directions are brought together and allowed to separate • Lighter liquid flows upward while the heavier liquid flows downward • Extract is the exit solvent rich stream containing the desired extracted solute • Raffinate is the exit residual stream containing little solute In solvent extraction, a raffinate is a liquid stream that remains after the extraction with the immiscible liquid to remove solutes EQUILIBRIUM RELATIONS IN EXTRACTION component A = solute component B = inert/carrier liquid component C = liquid solvent TERNARY DIAGRAM PLOTTING TERNARY DIAGRAM PLOTTING Acetic acid 100 95 90 85 80 75 70 65 wt% Acetic acid 60 55 50 45 40 35 30 25 20 15 10 5 Isopropyl 0 0 ether 5 10 15 20 25 30 35 40 45 50 55 wt% water 60 65 70 75 80 85 90 95 100 Water TERNARY DIAGRAM PLOTTING Acetic acid 100 95 90 85 80 75 70 65 wt% Acetic acid 60 55 50 Step 1: Plotting liquid-liquid equilibrium line for 3 components using data above. 45 40 35 Step 2: Create equilibrium tie-line by connecting a line between exact and raffinate points. 30 25 20 15 10 5 0 Isopropyl ether 0 5 10 15 20 25 30 35 40 45 50 55 wt% water 60 65 70 75 80 85 90 95 100 Water Example 7.1 Acetic acid 100 95 90 85 80 75 70 65 wt% Acetic acid 60 55 50 45 40 35 30 25 20 15 M 10 5 0 Isopropyl 0 ether 5 10 15 20 25 30 35 40 45 50 55 wt% water 60 65 70 75 80 85 90 95 100 Water Example 7.1 Acetic acid 100 95 90 85 To determine composition on A, B, C for extract & raffinate: Extend the line (equilibrium tie-line) pass M point, read the values (A, B, C) when at intersect between tie-line & extract and raffinate. 80 75 70 65 wt% Acetic acid 60 55 50 45 40 35 Extract 30 Xc = 0.94 25 XA = 0.04 20 XB = 0.02 15 M Raffinate Xc = 0.02 XA = 0.13 XB = 0.85 x 10 5 x 0 Isopropyl 0 ether 5 10 15 20 25 30 35 40 45 50 55 wt% water 60 65 70 75 80 85 90 95 100 Water SINGLE-STAGE EQUILIBRIUM EXTRACTION Acetic acid 100 The composition of the two equilibrium layer in Example 7.1 are for the extract layer (E) 𝑦𝐴 = 0.04, 𝑦𝐵 = 0.02, 𝑦𝐶 = 0.94, and for the raffinate layer (R) 𝑥𝐴 = 0.12, 𝑥𝐵 = 0.86, and 𝑥𝐶 = 0.02. The original mixture contained 100 kg and 𝑥𝐴𝑀 = 0.10. Compute the amounts of E and R. 95 90 85 80 E 75 70 65 wt% Acetic acid 60 R 55 50 Overall mass balance: E+R=M 45 40 35 30 A balance: E𝑦𝐴 + 𝑅𝑥𝐴 = 𝑀𝑥𝐴𝑀 25 20 15 M 𝑥𝐴𝑀 10 5 x x 0 Isopropyl 0 ether 5 10 15 20 25 30 35 40 45 50 55 wt% water 60 65 70 75 80 85 90 95 100 Water SINGLE-STAGE EQUILIBRIUM EXTRACTION Acetic acid 100 The composition of the two equilibrium layer in Example 7.1 are for the extract layer (E) 𝑦𝐴 = 0.04, 𝑦𝐵 = 0.02, 𝑦𝐶 = 0.94, and for the raffinate layer (R) 𝑥𝐴 = 0.12, 𝑥𝐵 = 0.86, and 𝑥𝐶 = 0.02. The original mixture contained 100 kg and 𝑥𝐴𝑀 = 0.10. Compute the amounts of E and R. 95 90 85 80 E 75 70 65 wt% Acetic acid 60 R 55 50 45 Overall mass balance: V + L = M =100 40 35 30 A balance: 𝑉(0.04) + 𝐿 0.12 25 20 15 M 𝑥𝐴𝑀 10 5 Solving, E = 75.0; R=25.0 x x 0 Isopropyl 0 ether 5 10 15 20 25 30 35 40 45 50 55 wt% water 60 65 70 75 80 85 = 100(0.10) 90 95 100 Water Level-Arm Rule SINGLE-STAGE EQUILIBRIUM EXTRACTION Determine E & R, using LEVER-ARM RULE Acetic acid 100 The composition of the two equilibrium layer in Example 7.1 are for the extract layer (E) 𝑦𝐴 = 0.04, 𝑦𝐵 = 0.02, 𝑦𝐶 = 0.94, and for the raffinate layer (R) 𝑥𝐴 = 0.12, 𝑥𝐵 = 0.86, and 𝑥𝐶 = 0.02. The original mixture contained 100 kg and 𝑥𝐴𝑀 = 0.10. Compute the amounts of E and R. 95 90 85 80 E 75 70 65 wt% Acetic acid 60 RR 55 50 45 Lever-arm rule: 𝐸 𝑅𝑀 Raffinate = Layer, R 𝑅 𝐸𝑀 40 Extract 35 Layer, E 30 25 20 15 M 𝑥𝐴𝑀 10 5 x x 0 Isopropyl 0 ether 5 10 15 20 25 30 35 40 45 50 55 (𝑉𝑀) ̅ wt% water 𝑉𝑀=12cm 60 65 70 75 80 85 90 95 100 Water SINGLE-STAGE EQUILIBRIUM EXTRACTION Acetic acid 100 The composition of the two equilibrium layer in Example 7.1 are for the extract layer (E) 𝑦𝐴 = 0.04, 𝑦𝐵 = 0.02, 𝑦𝐶 = 0.94, and for the raffinate layer (R) 𝑥𝐴 = 0.12, 𝑥𝐵 = 0.86, and 𝑥𝐶 = 0.02. The original mixture contained 100 kg and 𝑥𝐴𝑀 = 0.10. Compute the amounts of E and R. 95 90 85 80 E 75 70 65 wt% Acetic acid 60 R 55 50 Lever-arm rule: 𝐸 𝑅𝑀 = Raffinate 𝑅 𝐸𝑀 Layer, R 45 40 Extract 35 Layer, E 30 25 20 15 M 𝑥𝐴𝑀 10 5 x x 0 Isopropyl 0 ether 5 10 15 20 25 30 35 𝑉𝑀 40 45 50 55 (𝑉𝑀) ̅ wt% water 60 65 70 75 80 85 𝐿𝑀 𝐿𝑀=5.2cm 90 95 100 Water SINGLE-STAGE EQUILIBRIUM EXTRACTION Acetic acid 100 95 The composition of the two equilibrium layer in Example 7.1 are for the extract layer (E) 𝑦𝐴 = 0.04, 𝑦𝐵 = 0.02, 𝑦𝐶 = 0.94, and for the raffinate layer (R) 𝑥𝐴 = 0.12, 𝑥𝐵 = 0.86, and 𝑥𝐶 = 0.02. The original mixture contained 100 kg and 𝑥𝐴𝑀 = 0.10. Compute the amounts of E and R. 90 85 80 75 E 70 65 R wt% Acetic acid 60 55 50 Lever-arm rule: 𝐸 𝑅𝑀 = Raffinate 𝑅 𝐸𝑀 Layer, R 45 40 Extract 35 Layer, E 30 25 𝐸 𝑅𝑀 = 𝑀 𝐸𝑅 12 E= 100 17.2 = 69.77 20 15 M 𝑥𝐴𝑀 10 5 x x 0 Isopropyl 0 ether 5 10 15 20 25 30 35 40 45 50 55 wt% water 60 65 70 75 80 85 90 95 100 Water Example 7.2 Pure solvent isopropyl ether at the rate of 600 kg/h is being used to extract an aqueous solution of 200 kg/h containing 30 wt% acetic acid concentration by counter-current multistage extraction. The desired exit acetic acid concentration in the aqueous phase is 4%. Use the equilibrium data in A.3-24, compute the compositions and amounts of extract and raffinate. Example 7.2 (solution) 1. Write down the known points: Feed (F) Solvent (S) 200 kg/h 600 kg/h Acetic acid 30% - Water 70% - 𝑥𝑤 - 100% 𝑥𝐼𝐸 Isopropyl ether Raffinate (R) Extract (E) 4% 𝑥𝐴𝐴 Mixture (M) Example 7.2 (solution) 2.Locate the F, R, and S point on the ternary diagram. Acetic acid 100 95 3.Using the Lever rule to locate mixing point (M): 90 𝐹𝑆 = 𝐹𝑀 + 𝑀𝑆 = 10.7cm 85 80 𝑆 𝐹𝑀 = 𝐹 𝑀𝑆 75 70 65 wt% Acetic acid 60 600 10.7 − 𝑀𝑆 = 200 𝑀𝑆 55 50 45 𝑀𝑆 = 40 35 x 30 F 10.7 = 2.675 𝑐𝑚 4 25 20 15 M 10 x 5 S0x Isopropyl 0 ether x 5 10 15 20 25 30 35 40 45 50 55 wt% water 60 65 70 75 80 85 90 95 R 𝑦𝐴𝐴 = 0.04 𝑦𝑤 = 0.94 𝑦𝐼𝐸 = 0.02 100 Water Example 7.2 (solution) 4. Draw a straight line connecting point R to point M and extended to Extract layer. Acetic acid 100 95 90 85 80 75 70 65 wt% Acetic acid 60 55 50 45 40 35 x 30 F 25 20 15 10 M Ex x 5 S0x Isopropyl 0 ether x 5 10 15 20 25 30 35 40 45 50 55 wt% water 60 65 70 75 80 85 90 95 R 100 Water Example 7.2 (solution) 5. Read off 𝑥𝐴𝐴 = 0.08 at point E. Acetic acid 100 6. Mass balance: 95 90 Overall balance: F + S = E + R 800 = E + R 85 80 75 Acetic acid balance: (0.3)(200) + 0 = 0.08E + 0.04R R=100 kg/h E= 700 kg/h 70 65 wt% Acetic acid 60 55 50 45 40 35 x 30 F 25 20 15 10 M Ex x 5 S0x Isopropyl 0 ether x 5 10 15 20 25 30 35 40 45 50 55 wt% water 60 65 70 75 80 85 90 95 R 100 Water Example 7.2 (solution) 5. Using the lever rule to find R and E: Acetic acid 100 𝑅 𝐸𝑀 2.1 = = = 0.1935 𝐸 𝑀𝑅 10.85 95 90 85 R=0.1935E 80 Overall balance: F+S=R+E 800=0.1935E+E E=670.297 kg/h R=0.1935(670.297)=129.702 kg/h 75 70 65 wt% Acetic acid 60 55 50 45 40 35 x 30 F 25 20 15 10 M Ex x 5 S0x Isopropyl 0 ether x 5 10 15 20 25 30 35 40 45 50 55 wt% water 60 65 70 75 80 85 90 95 R 100 Water Number of Stages in Counter-current Extraction Example 7.3 Pure isopropyl ether of 450 kg/h is being used to extract an aqueous solution of 150 kg/h with 30 wt% acetic acid by countercurrent multistage extraction. The exit acid concentration in the aqueous phase is 10 wt%. Compute the number of stages required, the amount of 𝑬𝟏 and 𝑹𝑵 . Example 7.3 (solution) 1. Write down the known points: Feed (F) Solvent (S) 150 kg/h 450 kg/h Acetic acid 30% - Water 70% - 𝑥𝑤 - 100% 𝑥𝐼𝐸 Isopropyl ether Raffinate (𝑹𝑵 ) Extract (𝑬𝟏 ) 10% 𝑥𝐴𝐴 Mixture (M) Example 7.3 (solution) 2.Locate the F, 𝑅𝑁 , and S point on the ternary diagram. Acetic acid 100 95 3.Using the Lever rule to locate mixing point (M): 90 𝐹𝑆 = 𝐹𝑀 + 𝑀𝑆 = 10.7cm 85 80 𝑆 𝐹𝑀 = 𝐹 𝑀𝑆 75 70 65 wt% Acetic acid 60 450 10.7 − 𝑀𝑆 = 150 𝑀𝑆 55 50 45 𝑀𝑆 = 40 35 x 30 F 𝑹𝑵 𝑦𝐴𝐴 = 0.100 𝑦𝑤 = 0.875 𝑦𝐼𝐸 = 0.025 25 20 15 M 10 10.7 = 2.675 𝑐𝑚 4 x x 5 S0 x Isopropyl 0 ether 5 10 15 20 25 30 35 40 45 50 55 wt% water 60 65 70 75 80 85 90 95 100 Water Example 7.3 (solution) Acetic acid 100 4.Draw a straight line connecting point 𝑅𝑁 and M extending to extract layer 𝐸1 95 90 85 80 75 70 65 wt% Acetic acid 60 55 50 45 40 35 x 30 F 25 20 15 M 10 𝑬𝟏 5 x x x 𝑹𝑵 S0 x Isopropyl 0 ether 5 10 15 20 25 30 35 40 45 50 55 wt% water 60 65 70 75 80 85 90 95 100 Water Example 7.3 (solution) 5.Locate the operating point Δ. Acetic acid 100 95 90 85 80 75 70 65 wt% Acetic acid 60 55 50 45 40 35 x 30 F 25 20 15 x 10 𝑬𝟏 Δ x 5 𝑹𝑵 x S0 x Isopropyl 0 ether 5 10 15 20 25 30 35 40 45 50 55 wt% water 60 65 70 75 80 85 90 95 100 Water Example 7.2 (solution) Acetic acid 5. Draw a tie line from 𝐸1 to raffinate layer 𝑅1 100 95 6. Draw a line to connect 𝑅1 to Δ. 90 7. Draw a tie line from 𝐸2 to raffinate layer 𝑅2 85 80 8. Draw a line to connect 𝑅2 to Δ. 75 70 9. Draw a tie line from 𝐸3 to raffinate layer 𝑅3 (Exceeded 𝑅𝑁 ). 65 wt% Acetic acid 60 55 10. Number of stages 50 = number of tie lines ≅ 𝟐. 𝟑 45 40 35 x 30 F 25 20 x 𝑹𝟏 𝑹𝟐 𝑹 15 Δ x 𝑬𝟏 𝑬𝟐10 x 𝑬𝟑 5 x S0 x Isopropyl 0 ether xx 𝑵 𝑹𝟑 5 10 15 20 25 30 35 40 45 50 55 wt% water 60 65 70 75 80 85 90 95 100 Water Example 7.3 (solution) 𝑥𝐴𝐴 = 0.07 Mass balance: Overall balance: F + S = E1 + RN 150 + 450 = E1 + RN Acetic acid balance: (0.3)(200) + 0 = 0.07E1 + 0.10RN RN=100 kg/h E1= 500 kg/h MINIMUM SOLVENT RATE An aqueous feed solution of 150 kg/h with 30 wt% acetic acid is extracted using pure isopropyl ether. The exit acid concentration in the aqueous phase is 2.5 wt%. Compute the minimum solvent rate required to achieve this separation. Acetic acid 100 95 90 85 80 1. Draw a straight line connecting S and F. 75 70 65 wt% Acetic acid 60 55 50 45 40 35 x 30 F 25 20 15 10 5 x S0 x Isopropyl 0 ether 5 10 15 20 25 30 35 40 45 50 55 wt% water 60 65 70 75 80 85 90 95 𝑹𝑵 100 Water MINIMUM SOLVENT RATE An aqueous feed solution of 150 kg/h with 30 wt% acetic acid is extracted using pure isopropyl ether. The exit acid concentration in the aqueous phase is 2.5 wt%. Compute the minimum solvent rate required to achieve this separation. Acetic acid 100 95 90 85 80 2. Draw a tie line connecting F to intercepting the extract layer 75 70 65 wt% Acetic acid 60 55 50 45 40 35 x 30 F 25 20 𝑬𝒎𝒂𝒙 M 15 10 𝑹𝑵 5 x S0 x Isopropyl 0 ether 5 10 15 20 25 30 35 40 45 50 55 wt% water 60 65 70 75 80 85 90 95 100 Wate 3. Using lever rule: 𝐹𝑆 = 𝐹𝑀 + 𝑀𝑆𝑚𝑖𝑛 =9.4 cm 𝑆𝑚𝑖𝑛 𝐹𝑀 = 𝐹 𝑀𝑆𝑚𝑖𝑛 𝑆𝑚𝑖𝑛 = 9.4 − 3.8 × 150 = 𝟐𝟐𝟏. 𝟎𝟓𝟑 𝒌𝒈/𝒉 3.8 THE END By: Dr. Ng Ching Yin ngcy@ucsiuniversity.edu.my 100 95 90 85 80 75 70 65 wt% Acetic acid 60 55 50 45 40 35 30 25 20 15 10 5 0 0 5 10 wt% Isopropyl ether 15 20 25 30 35 40 45 50 55 wt% water 60 65 70 75 80 85 90 95 100