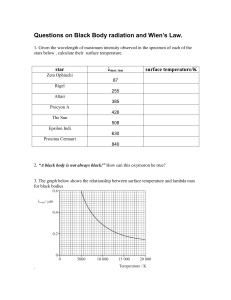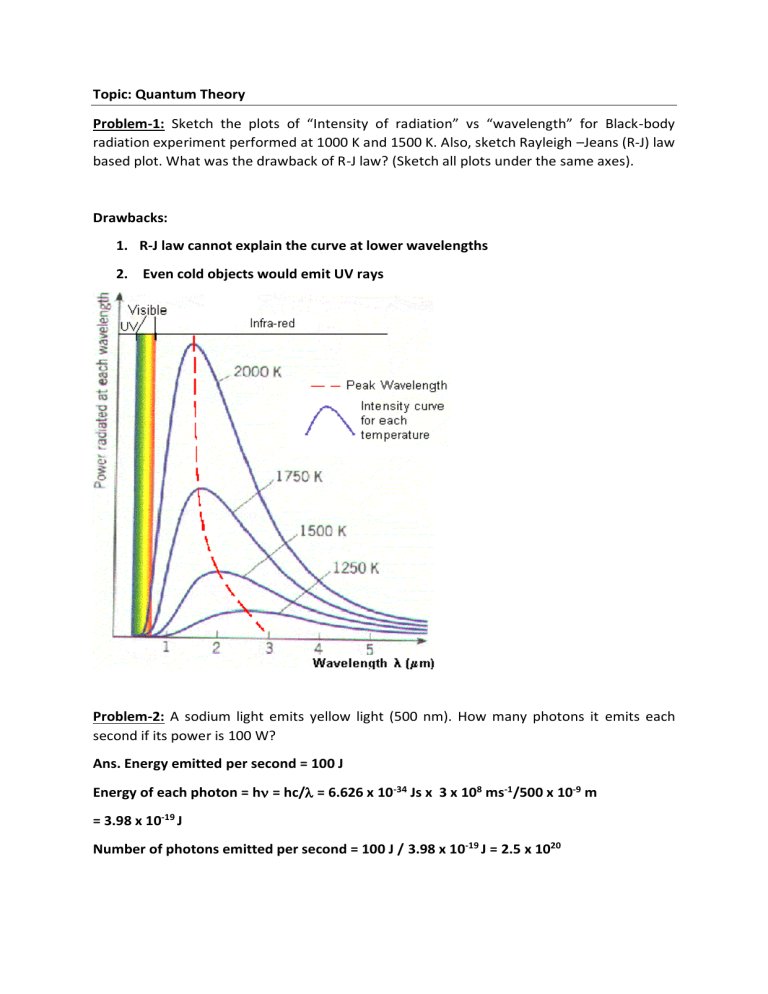
Topic: Quantum Theory Problem-1: Sketch the plots of “Intensity of radiation” vs “wavelength” for Black-body radiation experiment performed at 1000 K and 1500 K. Also, sketch Rayleigh –Jeans (R-J) law based plot. What was the drawback of R-J law? (Sketch all plots under the same axes). Drawbacks: 1. R-J law cannot explain the curve at lower wavelengths 2. Even cold objects would emit UV rays Problem-2: A sodium light emits yellow light (500 nm). How many photons it emits each second if its power is 100 W? Ans. Energy emitted per second = 100 J Energy of each photon = h = hc/ = 6.626 x 10-34 Js x 3 x 108 ms-1/500 x 10-9 m = 3.98 x 10-19 J Number of photons emitted per second = 100 J / 3.98 x 10-19 J = 2.5 x 1020 Problem-3: The maximum emission of the Sun occurs at λmax = 490 nm. What is it’s surface temperature? λmax.T = constant which is equal to 2.99 x 10-3 m.K 490 x 10-9 m x T = 2.99 x 10-3 m K T = 6102 K Problem-4: A glow worm emits red light of λ = 650 nm. If this light was a radiation from hot source, what would be the temperature? What do you conclude from your value? λmax.T = constant which is equal to 2.99 x 10-3 m.K 650 x 10-9 m x T = 2.99 x 10-3 m K T = 4600 K This is a photophysical/photochemical process and not a light emitted by a hot body. This formula is not applicable in this case. Problem-5: Calculate the power radiated by a blackbody at temperature 3000 K if this blackbody known to radiate 0.4 W at 1500 K. Emittance (M) = Power/area of the emitter = a.T4 Or, P / Area = a.T4 M = a.T4 where a = 5.67 × 10−8 W.m-2.K-4 P1500K = 0.4 W = Area x a x (1500 K)4 P3000 K = Area x a x (3000 K)4 P3000 K / P1500K = P3000 K /0.4 W = (3000)4/(1500)4 Therefore, P3000 K = 0.4 W x 24 = 6.4 W