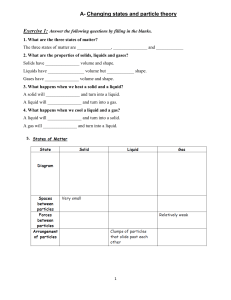
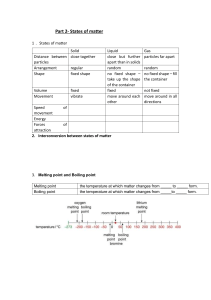
1/20/2024 States of Matter Dr. Martin Nasser What is Chemistry? the branch of science concerned with the substances of which matter is composed, the investigation of their properties and reactions, and the use of such reactions to form new substances. 1 1/20/2024 Syllabus: Cambridge IGCSE™ Chemistry 0620 Solids, liquids and gases: 1. State the distinguishing properties of solids, liquids and gases. 2. Describe the structures of solids, liquids and gases in terms of particle separation, arrangement and motion. 3. Describe changes of state in terms of melting, boiling, evaporating, freezing and condensing. 4. Describe the effects of temperature and pressure on the volume of a gas. 5. Explain changes of state in terms of kinetic particle theory, including the interpretation of heating and cooling curves. 6. Explain, in terms of kinetic particle theory, the effects of temperature and pressure on the volume of a gas. States of Matter Matter : is a substance that has a volume and a mass. Matter exists in three forms: Solids, liquids and gases. 2 1/20/2024 Comparison between Solids, Liquids and Gases Solid Liquid Gas Description Fixed volume, fixed shape. Fixed volume , takes shape of container. Any volume, takes shape of container. Arrangement of particles In a regular pattern called a “lattice”. Random Random Separation of particles Close together, touching. Still close together, just slightly further apart than in the solid phase. Separated , far apart. Movement of particles Vibration about a fixed position. Slow movement in a random way from place to place, sliding past each other. Fast random movement. Attractive forces between particles Stronger than in the liquid Slightly weaker than in phase. the solid phase. No attractive forces between particles. • Notes: Liquids and gases are able to flow but solids have fixed volumes and shapes. That’s why liquids and gases are called fluids. • Effects of changes in temperature: All three states show an increase in volume ( Expansion) when the temperature is increased and a decrease in volume ( contraction) when the temperature is lowered. The effect is much bigger for gases than solids and liquids. • Effects of changes in pressure: the volume of a gas at fixed temperature can be easily reduced by increasing the pressure on a gas. Gases are easily compressed. Liquids are only slightly compressible , and the volume of a solid is unaffected by changing the pressure. 3 1/20/2024 Changes in states of matter Important definitions: • Melting: the process that occurs when a solid turns into a liquid. • Melting point: the temperature at which a substances a solid turns into a liquid. It has the same value as the freezing point. Each pure substance has a specific melting point. • Boiling :the process that occurs when a liquid turns into a gas at the boiling point of the substance; a condition under which gas bubbles are observed from within the liquid and not just from its surface. • Boiling point: the temperature at which a substance boils. Each pure substance has a specific boiling point. 4 1/20/2024 Important definitions: • Evaporation: the process that occurs at the surface of a liquid as it turns into a gas. Evaporation can occur at temperatures lower than the boiling of a liquid. Its is affect by both surface area and the temperature of the liquid. 1. The larger the surface area the faster the liquid evaporates. 2. As the temperature increases the faster the liquid evaporates. • Volatility: the property of how easily a liquid evaporates. • Volatile: describes a liquid that evaporates easily. Liquid with a low boiling point evaporates more easily as they have weak intermolecular forces between the molecules in the liquid. Important definitions • Freezing: the process that occurs when a liquid turns into a solid. • Freezing point: the temperature at which a liquid turns into a solid. Pure substances have specific freezing point and it is equal to the melting point. • Condensation: the process that occurs when a gas turns into a liquid. • Sublimation: The process that occurs when a solid turns into a gas without turning into a liquid. Example Solid carbon dioxide which often called “dry ice”, when they are heated at normal pressure changes directly into gas. 5 1/20/2024 Heating curve: Notes: Changing of state is a physical change ( not a chemical change) On heating, the temperature rises until the solid starts to melt. However, close observation shows that the temperature stays constant until all solid has melted. The temperature then rises as the liquid warms further. On additional heating, the liquid reaches the boiling point were the liquid turns into gas and the temperature stays constant during the boiling point until all liquid has evaporated. Cooling Curve 6 1/20/2024 Kinetic theory When heat energy is given to a solid, the heat energy causes the particles to vibrate faster about a fixed position until the particles have sufficient energy for melting to occur. At the melting point, the energy gained by the particles is sufficient to overcome the attraction between particles in the solid. The ordered arrangement of particles then breaks down as the solid turns into a liquid. As this is occurring, there is no further increase in temperature until the ordered arrangement has completely broken down and all the solid has turned into a liquid. The energy given to the particles then causes them to move faster from place to place until they have sufficient energy for boiling to occur. At the boiling point the energy gained by the particles is sufficient to completely overcome the attraction between them in the liquid state. The particles then move as far away from each other as possible as the forces of attraction between them are almost completely overcome. Again there is no increase in temperature until the liquid has turned completely into a gas. In the gaseous state, the gas particles gain more and more energy and move at increasing speeds. Purity of substances • Pure substances: consists of only one substance without any contaminating impurities. A pure substance melts and boils at definite temperatures. This means that we can us them to test the purity of a sample. They can also be used to identify unknown substances. • Impure substances: is a substance which is mixed with other unwanted substances. The unwanted substances are called impurities. Note that impurities decrease the melting point and increases the boiling point of a substance. 7 1/20/2024 Mixtures of substances • Mixtures: Two or more substances mixed together but not chemically combined – the substances can be separated by physical means. • Solution: Is formed when a substance (solute) dissolves into another substance (solvent) • Solute: The solid substance that has dissolved in a liquid (the solvent) to form a solution. • Solvent: the liquid that dissolves the solid solute to form a solution. Example water is the most common solvent but liquids in organic chemistry that can act as solvents are called organic solvents. Mixtures of substances 8 1/20/2024 Mixtures of substances • Suspension: a mixture containing small particles of an insoluble solid, or droplets of an insoluble liquid, spread (suspended) throughout a liquid. Example precipitation reaction. • Note: in solutions in which on liquid is dissolved in another the term miscible is used. Example, alcohol and water are completely miscible. • Alloys are mixtures of metals. They are made by mixing the liquid metals together before solidifying the alloy. Mixtures of substances • Solubility : a measure of how much of a solute dissolves in a solvent at a particular temperature. Note that the solubility of the solids increase with temperature. • Saturated solution: a solution that contains as much dissolved solute as possible at a particular temperature. Crystallization depends on when a saturated solution is cooled , the solution can hold less solute at the lower temperature and the solute crystallizes out. Note the solubility of gases decrease as the temperature increases. 9 1/20/2024 Diffusion Definition: Movement of particles from a region of high concentration to a region of low concentration is known as diffusion. Note that diffusion occurs in both liquids and gases. Diffusion does not occur in solids as particles in solid does not move from one place to another as they only vibrate in a fixed position. The rate of diffusion in liquids is much slower than in gases. Example of diffusion Liquid bromine is highly volatile . After a short time , the brown gas begins to spread throughout the jar and the jar becomes full of brown gas. 10 1/20/2024 Diffusion rate depends on: • Mass of particles: the rate of diffusion is inversely related to the mass of the particles i.e. as the molecular mass increases the diffusion is slower. • Temperature: the average speed of the particles increases with an increase in temperature . i.e. diffusion increases with the increase of temperature. Note that diffusion is extremely fast in vacuum as the diffusing particles have less other particles to stand in their way. Also note that diffusion in gases is faster than that of liquids as there is more intermolecular spaces in gas than in liquids. Diffusion in gases 11 1/20/2024 Diffusion in gases: the effect of mass of particles The speed at which a gas diffuse depends on the mass of the particles involved. At the same temperature, molecules that have a lower mass move, on average , faster than those with a higher mass. When fumes of ammonia and hydrochloric acid are allowed to diffuse at the same time a ring of white smoke (solid ammonium chloride NH4Cl(s)) is formed closer to the source of hydrochloric acid showing that ammonia diffuses faster. Calculation of molecular mass of Ammonia (NH3) =14+3= 17 a.m.u. Calculation of molecular mass of Hydrogen Chloride (HCl)=1+35.5= 36.5 a.m.u. Thanks 12