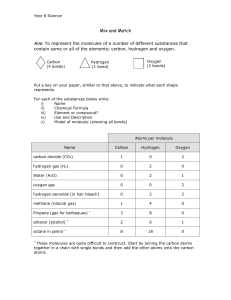
LECTURE #2 WATER AND CARBON ATOMIC STRUCTURE ● ● ● ● ● ● ● ● ● ● ● ● ● ● ● ● ● ● ● ● Matter: has mass and takes up space Atom: smallest chemical unit Neutron (it’s neutral = no charge): in the nucleus of an atom Proton (positive charge): in the nucleus of an atom Electron (negative charge): in orbits around nucleus Metre stick stretched to sun from earth: N / P = width of hair #Electrons = Protons normally Element: composed of a single type of atom Compound: composed of two or more types of atoms Atomic number = # of protons in nucleus (different atoms have different atomic numbers) Atomic mass = sum of protons Electrons normally ignored, so 1 atom = protons + neutrons (Electrons are very small compared to nucleus of an atom) 93 naturally occurring elements known Organisms normally utilize: 20 elements Isotopes: atoms of the same element with different numbers of neutrons. Carbon: ● 95% of C atoms have 6 protons and 6 neutrons (12C=carbon 12) ● 13C has 6 protons and 7 neutrons (= carbon 13) ● 14 C has 6 protons and 8 neutrons (= carbon 14 = very unstable) (The more neutrons the more unstable it gets) Unstable atoms release energy, protons, neutrons, electrons = radioactive decay (these isotopes are called radioactive isotopes) Nuclei determine type of element Electrons determine chemical behaviour. Electrons spin around nuclei in ‘shells’ or ‘clouds’ that depict the probable location of electrons at a given time (100 quadrillion times per second). ● More convenient to draw circles to represent shells / clouds. ● Each electron shell can hold only a certain number of electrons. ■ First shell has 2 maximum ■ Second has 8 maximum ■ Third has 8 maximum ● Possible to have more than 18 (in fourth shell), but not usually in life ● Outer orbits are stable when they have the full 8 electrons ● Outer orbits are unstable when they do not have all 8 (except Helium with 2) ● Valence electrons: outermost electrons in outer shell (valence shell) ● Valence: an atom’s ability to combine with other atoms ● Atoms combine with one another by: ● Sharing outer electrons (partial charges possible), or ● ● ● ● ● ● ● ● ● Transferring valence electrons (results in atom carrying full charge) ● Either of the above results in a ‘chemical bond Element: one type of atom (gold molecule, hydrogen molecule) Molecule: two or more atoms held together by a chemical bond Compound: two or more types of atoms held together (water) Each element has unique atomic number: Contains characteristic number of protons = atomic number. Mass number (indicated in superscript) = Number of protons + neutrons of most common isotope. Elements commonly found in organisms have at least one unpaired valence electron QUANTIFYING MOLECULES ● ● ● Molecular weight of a molecule ● = sum of the mass numbers of all the atoms in the molecule. One mole, or 6.022 ◊ 1023 molecules ● has a mass equal to the molecular weight expressed in grams. (NaCl = 58) Concentration of a substance in a solution: ● Typically expressed as molarity (M) ● = the number of moles per litre. CHEMICAL BONDS COVALENT BONDS: sharing of a pair of electrons by two atoms H+ + H+ = H2 fulfills the out shell; Sharing one pair of atoms = one covalent bond O++ + O++ = two covalent bonds; share two pairs of atoms The attraction of an atom for electrons is called: electronegativity The more the nucleus puts pressure on the electrons, the more electronegativity an atom has. Thus, the more protons in the nucleus, the more pull on the electrons (attraction of + of protons with the – charges of the electrons) The farther the electron is from the nucleus (in a more outer orbit), the less the electronegativity of the atom In atoms with equal electronegativities, the electrons tend to spend equal time around each atom and no ‘poles’ are formed. Thus these are called ‘nonpolar’ covalent bonds (like hydrogen, oxygen). Chemical formulas: H-H or H:H or H2 are all the same and known as structural formulas. ATOMIC STRUCTURE CHEMICAL BONDS L2 COVALENT BONDS REPRESENTING MOLECULES ● ● ● The shape of a simple molecule is governed by the geometry of its bonds. The molecular formula indicates the numbers and types of atoms in a molecule (e.g., H2O, CH4). Structural formulas indicate which atoms are bonded together and whether the bonds are single, double, or triple bonds. MOLECULAR REPRESENTATION CHEMICAL BONDS Carbon has four electrons in outer shell Thus needs four electrons to satisfy the outer shell requirement. E.g. C + O + 2H; C + 2O; C + 4H Since carbon has only four valence electrons Has a tendency to either lose four valence electrons or gain four valence electrons. Tend to share atoms and form chains. C–C–C–C–C Tend act as an intersection with latter groups; Leads to ‘backbone’ of many biological compounds (known as organic compounds [proteins, carbohydrates]). If two covalently bound atoms have significantly different electronegativity: Their shared atoms will spend more time with the more electronegative atom (more pull from the larger number of protons). Water is an excellent example of this (oxygen has much greater electronegativity than hydrogen atoms) Thus oxygen gets a transient (partial) negative charge. Each of the hydrogens get a transient (partial) positive charge. Because the oxygen has a partial negative charge (δ–) and the hydrogen have a partial positive charge (δ+), ‘poles’ have been created, and thus the bond is polar. Polar charges can form between many different elements Generally molecules with polar covalent bonds are water soluble, nonpolar are not. The most important polar bonds involve hydrogen because they allow hydrogen bonding (discussed shortly) ● An atom in a molecule with high electronegativity will hold the electrons more tightly and have a partial negative charge (δ–), whereas the other atom will have a partial positive charge (δ+). GREEK SYMBOLS USED IN BIOLOGY Αα Alpha Νν Nu * Ββ Beta Xi * Γγ Gamma Οο Omicron Δδ Delta Pi Εε Epsilon Ρρ Rho Ζζ Zeta Sigma Ξξ Ππ Σσς * Ηη Eta Ττ Θθ Theta Υυ Upsilon Ιι Iota Φφ Phi Κκ Kappa Χχ Chi Λλ Lambda Ψψ Μμ Mu Omega * Ωω Tau Psi * WATER MOLECULES AND HYDROGEN BONDING STRUCTURE OF WATER: LIQUID AND ICE IONIC BONDS: When an atom gives up or receives an electron (doesn’t share) as is the case of table salt (NaCl). Ionic bonds occur when two atoms have vastly different electronegativities (Na with e.n. of 0.9 and Cl with 3.0). Sodium with only one electron in valence shell has one less electron than # of protons (= +1 charge) and chlorine having 7 electrons in the valence shell is one electron shy of filling the valency shell (= -1 charge). Atoms with either a full negative charge or full positive charge are called ions. Therefore, the sodium ion with one positive charge and the chlorine with a full negative charge, attract each other forming a crystal lattice. Ions do not share electrons. Cation: An atom that loses an electron and becomes positively charged. ● ● Anion: An atom that gains an electron and becomes negatively charged. Often called salts when it dissociates in water and forms cations / anions. IONIC BONDS CHEMICAL BONDS Partial negative charge of oxygen attracts positive ions and the partial negative ions; the presence of hydrogen bonds interferes with the attraction of the cation with anion. When cations and anions dissociate from one another and become surrounded by water molecules (they are hydrated), they are called electrolytes because they can conduct electricity though the solution. Electrolytes are critical to life because: - They stabilize a variety of compounds - Act as electron carriers Allow electrical gradients to exist within cells HYDROGEN BONDS Hydrogen bond: an electrical attraction between a partially charged hydrogen atom and a full or partial charge on either a different region of the same molecule or on a different molecule. Hydrogen bonds are generally weak Hydrogen bonds are not covalent bonds as they don’t share electrons Hydrogen bonds are essential for life Stabilize three dimensional shapes of large molecules (DNA) Exact shapes of molecules are essential for function (enzymes, antibodies, intercellular chemical messengers, recognition of target cells by pathogens). Can be overcome when necessary as they are fairly weak (unzipping DNA during replication). PROPERTIES OF WATER ● ● ● ● ● Life is based on water because water is a great solvent. Hydrogen bonding makes it possible for almost any charged or polar molecule to dissolve in water Covalent bonds in water are polar because oxygen and hydrogen differ in their electronegativity: oxygen has a partial negative charge and hydrogen has a partial positive charge. Ions and polar molecules stay in solution because of their interactions with water’s partial charges. Thus: hydrogen bonds are extremely important in biology. STRUCTURE OF WATER ● Water also has several striking physical properties: (1) Expands as it changes from a liquid to a solid ● Thus water is denser as a liquid than as a solid: Ice: Hydrogen bonds connect water molecules in an open crystal pattern. Liquid: there are fewer hydrogen bonds and the water molecules can pack more closely together. (2) It has an extraordinarily large capacity for absorbing heat (energy). (i.e., a very high specific heat and heat of vaporization.) (3)Water is cohesive and has high surface tension Binding between molecules is called cohesion. Binding between molecules is called adhesion. HOW MANY BONDS CAN AN ATOM HAVE? ● ● ● ● The number of unpaired electrons determines the number of bonds an atom can make. Atoms with more than one unpaired electron can form multiple single bonds or double or triple bonds. Molecules form when atoms bond to each other. Most of the important compounds in organisms contain carbon. ACID-BASE REACTIONS AND pH ● ● ● ● Puissance de hydrogene (power or potential of hydrogen) In acid–base reactions, a proton donor (acid) transfers a proton to a proton acceptor (base). The pH scale is logarithmic: pH = −log [H+]. The pH of pure water is 7. pH indicates the concentration of protons in solution. ● The concentration of H protons in a solution: ● = log rhythm of -7 (10 to the -7 power [10-7]) ● = 1/10,000,000 of all the molecules in the solution at a pH of 7 pH is the negative of a log, therefore, a negative of a negative = 7 A pH of 6 is equal to 1/1,000,000 or 10 to the -6 power [10 -6] A pH of 1 is equal to 1/10 or 10 to the -1 power [10 -1] or one out of every 10 molecules in solution is a proton A pH of 14 is equal to 1/100,000,000,000,000 (10 -14) pH ● ● ● ● ORGANISM TOLERANCE FOR pH ● ORGANISMS can only tolerate a certain, relatively narrow pH range. ● Cyanobacteria grow well in basic environments ● Fungi tend to do well in acidic environments ● Some bacteria do well in acidic environment, e.g. Heliobacter pylori (causes ulcers) ● Microorganisms can change the pH of their environment by: ● Utilizing acids and/or bases, and ● Through elimination of waste products. LECTURE #3 PROTEINS AND NUCLEIC ACIDS Definitions: Penetrate: Succeed in forcing a way into or through. Receptor: Receptor, molecule, generally a protein, that receives signals for a cell. Central carbon atom: Each molecule contains a central carbon (C) atom, called the α-carbon, to which both an amino and a carboxyl group are attached. Coalesce: Mix Polymerization: reactions require energy and are not spontaneous. Hydrolysis: the chemical breakdown of a compound due to reaction with water. Condensation Reactions: Condensation reaction is the combination of two molecules to form a single molecule Condensation: water which collects as droplets on a cold surface when humid air is in contact with it.: "the inside of the cab steamed up with condensation". Dipoles: An electric dipole deals with the separation of the positive and negative charges found in any electromagnetic system Molecular chaperones: Auxiliary proteins that protect and stabilize folding proteins. Denatured protein: Denaturation involves the breaking of many of the weak linkages, or bonds (e.g., hydrogen bonds), within a protein molecule that are responsible for the highly ordered structure of the protein in its natural (native) state. What do proteins do? Structural: Within cells and within the organism. The linear sequence of amino acids within a protein. Contractile(capable of…): movement Signals: transportation from one organ to other cells in the body. Receptors: on the cell surface: correct configuration to receive the signals Membrane transport: transport signals across the cell membrane Antibodies: bind up antigens (e.g. viruses) so can’t penetrate cells. Enzymatic: makes molecules react = metabolism = life All proteins are made from just 20 amino acids. What is an amino acid molecule? - A central carbon atom that bonds to - NH2, (Amino) - COOH, (Carboxyl) - H, (Hydrogen) - a variable side chain (represented by the letter R) The 20 amino acids differ only in the variable side chain (R-group) attached to the central carbon NH2-C-COOH (backbone) ● R-groups differ in their size, shape, reactivity, and interactions with water. (1) Nonpolar R-groups: Do not form hydrogen bonds; coalesce in water (2) Polar R-groups: Form hydrogen bonds; readily dissolve in water How Amino Acids Interact with Water Condensation and Hydrolysis Reactions Amino acids polymerize to form peptides; then into proteins. - Polymerization reactions require energy and are not spontaneous. Monomers polymerize (many monomers linked together) through: ○ Condensation Reactions (dehydration synthesis), which releases a water molecule Polymers are de-polymerized by the reverse reaction, hydrolysis, water reacts with a polymer to release a monomer (lyses the peptide). The Peptide Bond ● ● Condensation reactions bond ● Carboxyl group of one amino acid bonds to the amino group of another to form a peptide bond by releasing a water molecule. A polypeptide ● Is flexible and ● Is directional ● The N-terminus has a free amino group ● C-terminus has a free carboxyl group, and ● ● Side chains extend out from the backbone. ● R ● | NH2 – C - COOH Peptide Bond Formation: What do Proteins look like? ● ● Proteins are diverse in size and shape, as well as in the chemical properties of their amino acids. Proteins have four basic levels of structure: ● Primary structure: amino acid polymers (peptides) ● Secondary structure: folding of amino acid polymers (proteins) ● Tertiary structure: three dimensional folding: hydrogen and sulfur bonds ● Quaternary structure: more than one amino acid polymers clustered together Primary Structure ● ● ● A protein’s primary structure is its unique sequence of amino acids polymers (called residues) May consist of many different amino acids and in length. Because the amino acid R-groups affect a polypeptide’s properties and function: ● Just a single amino acid change can radically alter protein function (altered protein in cows > mad cow disease). Secondary Structure: ● ● ● Secondary structure results in part from: ● Ionic bonds ● Hydrogen bonds ● A polypeptide must bend to allow this hydrogen bonding—thus, α-helices or β-pleated sheets are formed. Secondary structure depends on the primary structure— ● Some amino acids are more likely to be involved in α-helices; ● Others, in β-pleated sheets. Secondary Structure increases stability by way of the large number of hydrogen bonds. Hydrogen bonds form between peptide chains. Secondary structures of proteins result. Α-helices β-pleated sheets Ribbon diagrams of secondary structure. Tertiary Structure - The tertiary structure of a polypeptide results from interactions between R-groups or between R-groups and the peptide backbone. - These contacts cause the backbone to bend and fold, and contribute to the 3D shape of the polypeptide. - R-group interactions include hydrogen bonds, covalent disulfide bonds, and ionic bonds, van der Waals interactions. Hydrogen bonds can form between hydrogen atoms and the carboxyl group in the peptide-bonded backbone, and between hydrogen atoms and atoms with partial negative charges in side- chains. - - Covalent bonds between sulfurs of the amino acid cysteine are crucial to maintain tertiary structure. Are not repetitive like the a-helices and b-pleated sheets Van der Waals Forces The forces of attraction which hold an individual molecule together (for example, the covalent bonds) are known as intramolecular attractions. ● ‘Van der Waals forces’ are Intermolecular attractions: attractions between one molecule and a neighbouring molecule. ● Temporary polarity due to the location of an electron at any one moment. Unequal sharing of electrons causes rapid polarization and counter-polarization of the electron cloud forming short lived dipoles in molecules. These dipole interact with the electron clouds of neighboring molecules forming more dipoles. ● ● ● These forces weaker than other bonds Although these interactions are weak, the large number of van der Waals interactions in a polypeptide significantly increases stability. Ionic bonds form between groups that have full and opposing charges. TERTIARY STRUCTURES OF PROTEINS Quaternary Structure Some proteins contain several distinct polypeptide subunits that interact to form a single structure; - The bonding of two or more subunits produces quaternary structure. The combined effects of primary, secondary, tertiary, and sometimes quaternary structure allow for amazing diversity in protein form and function. Folding and Function Protein folding is often spontaneous because: - The hydrogen bonds and van der Waals interactions make the folded molecule more stable energetically than the unfolded molecule. Proteins called molecular chaperones help proteins fold correctly in cells. Can combine with other types of molecules for other functions: - For example: Glycoproteins on the cell membrane surface (receptors). Because shape determines function, anything that alters shape has the potential to disrupt function (denatured). - A denatured protein is unable to function normally when: - Amino acid substitution - Heat - Changes in pH - Salt concentration - All the above can interfere with hydrogen and ionic bonding An Intro to Catalysts A catalyst: may be the most fundamental of protein functions. Reactions take place when: - Reactants collide in precise orientation - Enzymes bring substrates together in precise orientation so that the electrons involved in the reaction can interact. - Enzymes usually catalyze only one reaction (active site made for one type of molecule) - - Substrates bind to the enzyme’s active site, and interactions between the enzyme and the substrate stabilize the transition state and lower the activation energy required for the reaction to proceed. Most biological chemical reactions occur at meaningful rates only in the presence of an enzyme. Enzyme catalysis has three steps: (1) Initiation: reactants are precisely oriented as they bind to the active site. (2) Transition state facilitation: interactions between the substrate and active site R-groups in the enzyme lower the activation energy. (3) Termination: reaction products are released from the enzyme. Do Enzymes Act Alone? Some enzymes require cofactors to function normally. These are either metal ions or small organic molecules called coenzymes. Most enzymes are regulated by molecules that are not part of the enzyme itself. - Competitive inhibition occurs when a molecule similar in size and shape to the substrate competes with the substrate for active site binding. - Allosteric regulation occurs when a molecule causes a change in enzyme shape by binding to the enzyme at a location other than the active site. What do proteins do? Slides 36-42 LECTURE #4 - Nucleic Acids Nucleic acid is: ● ● ● ● ● polymer of nucleotides each composed of phosphate group, sugar, nitrogenous base sugar is ribose in: RNA deoxyribose in: DNA (missing an oxygen) Nucleotides are polymerize Nature of DNA’s secondary structure: Erwin Chargaff: two empirical rules for DNA: (1) –total number of purines equals the number of pyrimidines (2) –numbers of: A = T -numbers of: G = C Watson and Crick determined (early 1950’s): (1) Antiparallel DNA strands form a double helix - NITROGENOUS BASES FACED INWARD, NOT OUTWARD -(2) DNA strands form: DNA IS A DOUBLE HELIX: DNA’S Secondary Structure: How does DNA replicate? ● ● Complementary base pairing provides simple mechanism for DNA replication Requires three steps: (1) Separate of the DNA strands (2) Breaking the hydrogen bonds between the nitrogenous bases (3) Pair up the bases of the nucleotides to form a complementary strand Is DNA a Catalytic Molecule? ● DNA’s stability makes it a reliable store for genetic information ● Because DNA does not appear to be able to catalyze any chemical reaction, biologists think the first life-form was made of RNA, not DNA. ● DNA less reactive than RNA and more stable (makes for a poor catalyst) and less susceptible to chemical degradation. RNA Structure and Function: DIFFERENCE IN STRUCTURE OF RNA AND DNA (1) uracil instead of thymine (2) contains ribose instead deoxyribose * Presence of the –OH group on ribose makes RNA much more reactive and less stable than DNA. RNA’s Secondary Structure: ● ● ● ● RNA’s secondary structure results from complementary base pairing (hairpin configuration) The bases of RNA typically form hydrogen bonds with complementary bases on the same strand. The RNA strand folds over, forming hairpin structure: RNA molecules can have: tertiary and quaternary structures SECONDARY STRUCTURE IN RNA: The first life form = RNA ● RNA can provide: ● - template of self and ● ● - complementary strand ● and is - quite reactive and act ● - as a catalyst RNA used in Pfizer and Moderna vaccines. LECTURE #5 LECTURE #6