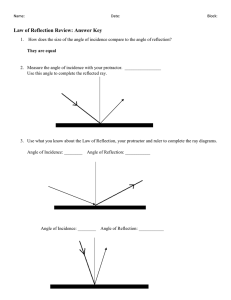
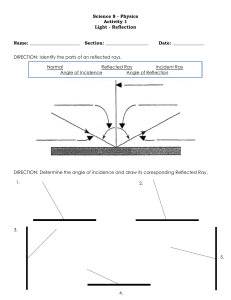
MY PHYSICS S.B.A Experiment #1 Title: Reflection Aim: To investigate the relationship between the angle of incidence and the angle of reflection. Theory: Reflection is said to occur when a light ray is bounced off a reflective surface. To measure the angles, the protractor is placed on the normal in line with the 900 line on the protractor and read from the 900 to the base line. The precautions associated with this experiment are to place the mirror on the line drawn and protractor on 900 line. Variables: manipulating variable: angle of incidence (c) responding variable: angle of reflection (r) Apparatus: a sheet of paper, tacks, 4 optical pins, optical board, mirror, mirror support (block), protractor, sharp pencil. Diagram: Method: a sheet of paper was tacked onto a board. A mirror line was then drawn close to the edge of the paper. A protractor was used to draw in a normal in the midst of the mirror line. The protractor was used again to draw lines at the angle of incidence from 150, 300,750. The mirror was then placed on the line so that the silver edge was on the line drawn. Two pins were placed on the 1ST line of incidence (150) Pin A was close to the edge of the paper. The images of the pin of pin A & B close to the mirror. A fourth pin (D) was paced at the edge of the paper in line with pin (C) and the images of pin A & B. All pins were removed and an (X) was placed over the hole left from the pins. The mirror line was extended and a line was drawn in to indicate the path of the reflected ray. The protractor was then used to measure the angle of reflection results were taken of it and its corresponding angle of incidence was repeat was done to all the other incidence line. Results: Table showing angle of incidence and reflection Ө ἱ l0±l0 10 20 30 40 50 60 Ө rl0±l0 10 20 30 40 50 60 Analysis: Reflection is the throw back of rays when they have reached a boundary or reflective surface. There are two laws governing reflection they state that: 1. The angle of incidence and angle of reflection are equal. This was shown when the angle of incidence and the angle of reflection were measured. All angles were equal. 2. The incident vary, reflected ray and the normal, all lie in the same place. This was proven as the incidence ray. Reflected ray and the normal could be drawn on a sheet of paper. Precautions: Plane mirror was held steadily on the sheet of paper. Protractor measurements were taken accurately. Angles were measured correctly. Lines were drawn correctly to the angles measured. Causes of error: A parallax error may have occurred with pins (1) and (2) resulting in wrong placement of pins (3) an error in the protractor reading may have occurred. Conclusion: the angle of incidence is equal to the angle of reflection: the incident, reflected rays and normal all lie in the same plane. Experiment #2 Title: Refraction Aim: To investigate the relationship between the angle of incidence and the corresponding angles of refraction for glass Objectives: Refraction is the bending of rays when travelled from one medium to another Variables: manipulating: angle of incidence responding: angle of refraction Apparatus: glass lock, ruler, paper, protractor, four coloured markers, tacks, pencil, board. Diagram: Procedures: A sheet of paper was tack onto a board. The glass block was placed in the middle (Center) of the paper and the outline of it was drawn with a pencil. The protractor was used to draw a normal on the middle of the long side of the block. The intersection of the normal and the glass block was labeled as point x. The protractor was use again to measure out and draw lines at the angle of incidence of 100,200, up to 600. On the line two lines were drawn upright, so that the line was close to the block as possible and line 1 was close to the edge of the paper. The glass block was then replaced. After looking through the glass block at image 1 and 2 the image of line 1 was directly behind the image of line 2. Line 3 was close to the block so it appears to be in line with images of line 1 and 2. Line 4 was drawn close to the edge of the paper in line with line 3 and the images of 1 and 2 was placed over the line after line 3 was measured with a protractor and then recorded. The procedure was repeated for all angles of incidence drawn. Table of incidence: table showing the relationship between angle of incidence and refraction Ө ἱ l0±l0 10 20 30 40 50 60 Ө rl0±l0 7 14 19 20 32 38 Sin ἱ 0.1736 0.3420 0.5 0.6427 0.7660 0.8660 Sin r 0.1045 0.2079 0.2923 0.4067 0.4848 0.5446 Precautions: Place protractor on the 900 line. Place glass block in box property. Sources of Error: systematic error in protractor parallax error in protractor readily and in drawing emergent ray. Conclusion: The ratio of the sine of the angle of incidence divided by the sine of the angle of refraction is a constant shown as the refractive index according to snells law, the refractive index of the glass block is 1.5 which was proven in the experiment. Experiment #3 Title: Planning and designing (Refraction) Problem Statement: plan and design and experiment to measure the refractive index of sea water near the shore Hypothesis: In sea water, a can appears clearer than it is. Aim: To measure the refractive index of sea water near the shore Apparatus: Coin, Ruler, Aquarium, Sea Water, Diagram: Method: 1) Set up apparatus as shown in the diagram 2) Use the ruler to locate and measure the distance of the image 3) Record the real depth and apparent depth of the coin 4) Repeat step 2&3 two more times and find the average of the apparent and real depth 5) Use the formula for real depth to find the refractive index apparent depth Expected Result: Real Depth 1M Apparent Depth 1M Refractive Index Treatment of results: plot graph of real depth is apparent depth Precautions: 1) Do not do the experiment on a rocky surface or windy area to prevent observation of wrong reading of the real and apparent depth Sources of Error: 1) Incorrect calculation 2) Windy area 3) Reading the ruler incorrectly Experiment #4 Title: Planning and designing (Pendulum) Problem Statement: A student propose that the mass at the end of the pendulum will affect the period of the pendulum Hypothesis: changing the mass on the string will not affect the time at which it takes for the pendulum to swing one complete oscillation Aim: to investigate that the mass at the end of the pendulum will affect the period of the pendulum Apparatus: stop watch, pendulum bob, and 3 different masses. Diagram: Method: 1) set up the apparatus 2) Attach a weight to the string and record the time taken for it to make 20 oscillations 3) Remove the previous weight and add another weight and record the time 4) Repeat step two with a different weight and record the time 5) Record result in appropriate table Expected Result: Weights 1st time 2nd time 3rd time Precautions: pendulum should hang vertical and be securely supported Sources of error: 1) Draft can cause an interruption in the reading of the pendulum in which it can increase or decrease it 2) Masses of the weight not being placed on the stand properly 3) Starting the stop watch at the wrong time Experiment #5 Title: Deformation Problem Statement: to determine the relationship between applied force and the resulting extension for a string Apparatus & Materials: Refort stand, paper clip, painter, six (6) 2g masses, 50cm ruler, steel spring, beaker (200ml), containing 200ml of water. Diagram of Apparatus: Variables: manipulated: mass added: applied force (N) Controlled: the string Responded: extension of the spring Method: the apparatus was set up as shown in the diagram. The reading of the pointer was noted before adding any weight. This was the initial pointer reading. A single 50g mass was added and the reading of the pointer was recorded. The difference in the pointer reading between the final and the initial, gave the extension of the spring. Another 50g mass was added and the pointer reading was recorded until the total mass was 300g. The clamp was adjusted to completely immerse the base of the mass holders in a beaker of water. The new initial reading of the pointer was recorded. The difference between the pointer readings gave the apparent extension of the string Result: Mass Added (g) Final Reading (cm) 24.4 29 31.4 35.8 39.7 44.5 48.5 0 50 100 150 200 250 300 Extension (cm) 0 5.4 7 11.4 15.3 20.1 24.1 Applied Force (N) 0 0.5 1.0 1.5 2.0 2.5 3.0 Final Reading: 31.5 cm Initial Reading: 29.9 Apparent Extension: 3 cm Data Analysis: A graph of force (N) against extension, ἱ, was plotted. Its gradient was then calculated using the formula M = (y2-y1)/(x2-x1) = (24.8-12) / (3.5-1.25) = 5.69 According to the graph, the apparent weight of the immersed 50g mass is 0.38N. The relationship between the applied force and the extension that 1 is proportional to 10. This is in accordance with Hookes law which states that the extension is directly proportional to the land provided that the elastic limit was not exceeded. Precautions: ensure the environment is constant in order to get correct results. Sources of error: 1) Inherit error in 50cm ruler 2) Parallel error in reading scale Conclusion: A straight line graph through the origin indicates that the extension of t he spring is directly proportional to the stretching force that was applied to it. Experiment #6 Title: To find the density of an irregular shaped object (Stone) Apparatus: Stone, measuring cylinder, water, balance Diagram: Procedures: The mass of the stone was found by using the balance. The stone was then placed in a measuring cylinder containing a known Volume of water. The new volume was recorded. The procedure was repeated until a consistent answer for the volume obtained. Result: Mass of Stone 13.35 13.35 13.35 Initial volume of water (cm3) 50 40 70 Final volume of water (cm3) 55 46 76 Data Analysis: The density of the stone was calculated Density = mass/volume = 13.35/5 = 2.67g/cm3 Precautions: (1) Ensure that the balance is zeroed (2) Ensure that the measuring has water Sources of error: (1) The balanced was not placed on a surface (2) The reading were not properly taken Conclusion: the density of the stone was found to be 2.67g/cm3 Density of stone g (cm3) 13.35/5=2.67 13.35/6=2.23 13.35/6=2.23 Experiment #7 Title: Heat Apparatus: To find the specific heat capacity of a metal using the method of mixtures. Diagram: Procedures: The mass ms, of the metal was found and recorded using a balance. A string was tied to the metal then placed in a beaker with water where the water was allowed to boil for at least 5 minutes. The mass ms of the liquid placed in the Styrofoam cup was found by using the balance to find the mass of the cup alone, and the mass of the cup and the liquid. The initial temperature q, of the liquid in the cup was recorded. The metal was transferred as quickly as possible from the beaker to the Styrofoam cup with liquid and string began. The highest temperature, 0, of the liquid obtained after, the metal placed in the Styrofoam cup was recorded. Data Analysis: The transfer of the metal should be done quickly as heat may be lost to the surrounding. Mixing causes the higher temperature to flow to the lower temperature until both substances are at the same temperature. The Styrofoam cup is a good choice to use in this experiment as the material making it up is a good insulator which means heat cannot be conducted by it. Heat energy Joined by liquid= heat energy lost by metal. Formula = .: MM X CM X Tm = M2 X C2 X T2 0.1KG X CM X (100 -34)J = 92.82 X 4200J X (34-28) 0.1KG X CM X 66J = 0.0928 X 4200 X 6 CM = (0.0928 X 4200 X 6)/ 0.1KG X 66J CM = 354.33 JKg-1K-1 The assumption is not true as most of the heat given off is loss to the atmosphere before it reaches the liquid. Precautions: (1) Do not put your hands near the flame (2) Ensure that the metal is transfer quickly Sources of error: (1) Thermometer might not be read correctly (2) The metal was not transfer quickly hence heat may be lost Conclusion: The specific heat capacity of a metal was found to be 354.33 JKg-1K-1 By using methods of mixture. Experiment #8 Title: planning and Design (Heat) Problem and statement: plan and design and experiment to investigate how concentration (amount of dissolve in liquid) affects the specific heat capacity of a liquid. Aim: To investigate whether the concentration of a salt solution affect the specific heat capacity of a solution Hypothesis: Increase in concentration of salt solution will increase the specific heat capacity of a liquid Variables: Manipulating: concentration of solution Responding: specific heat capacity Control: volume of water Apparatus: salt, water, beaker, metal, thermometer, stop watch, balance. Diagram: Procedures: (1) Pour 40cm3 of water in a beaker and record its temperature (2) Add 10g of sodium chloride (Nacl) to the water and record new temperature (3) Place the beaker over a heat source then record the time taken for boiling point (4) Repeat the process using various mass of salt (Nacl) 20g and 30g respectively (5) Record your results Expected Results: Mass of Salt 10g 20g 30g Temperature (00C) 1100C 1200C 1500C Precautions: (1) Ensure that the mass of the salt is accurate (2) Ensure that the same amount of water is poured in the beaker Sources of error: (1) Parallax error in reading the temperature may occur (2) The incorrect amount may have been placed in the beaker Experiment #9 Title: Center of Gravity Aim: To locate the center of gravity of an irregular shape lamina Apparatus: Material: clamped stand, needle/nail, lamina, string, stone. Diagram: Procedures: 1. The equipment was obtained and the experiment was set 2. It was ensured that the pivot (nail/optical, pin stuck in cork was stable by placing it in a stand or on the bench top) 3. A hole was placed close to the body and suspended on the pivot to ensure that it was spinning freely. 4. The plumLine was suspended on the pivot and when the lamina was in equilibrium the position of the plumLine was recorded at X. The X was placed as close as possible to the edge; a sharp pencil was used to mark the position of the plumLine. The steps were repeated for another position. Discussion: The center of gravity is the point through which the total weight of the body is considered to act Sources of error: (1) The diagram was affected by parallax (2) Lamina was not hang freely Conclusion: The position of the center of gravity was found and the aim was found. Experiment #10 Title: Pressure Aim: To investigate fluid pressure and depth Apparatus: Two 1 litre plastic bottle, nail water, tape, meter ruler. Diagram: Procedures: 1. A nail was used to punch three holes in the containers as shown in the diagram above 2. The containers were placed at the edge of the table and another container placed below to catch the water 3. The holes in the containers were taped. Water was poured in the containers until it passed the holes. The tape was removed and the flow of water from each hole of the container was observed 4. The water flow from each hole of the container was measured 5. The equipment was repeated with the containers covered Data Collected: TABLE SHOWING RESULTS OF CONTAINER 1 IN PRESSURE EXPERIMENT A B C Uncovered Container (cm) 3 6 8 covered Container (cm) 0 3 5 TABLE SHOWING RESULTS OF CONTAINER 2 IN PRESSURE EXPERIMENT A B C Uncovered Container (cm) 5 5 5 covered Container (cm) 4 4 4 Data Analysis: The pressure varied in container 1. In container 1 the pressure was highest at hole ‘C’ and lowest at hole ‘A’. Conclusion: From the experiment it was concluded that as depth increases pressure also increases