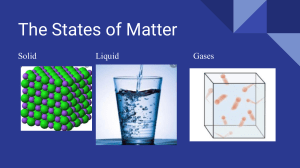
Name:_____________________________________________________ Grade &Section:_____________________________ States of Matter: Exploring Solids, Liquids, and Gases The world around us is composed of matter in three fundamental states: solid, liquid, and gas. These states differ in their properties due to the behavior of their particles. Solids are characterized by having a fixed shape and volume. The particles in a solid are tightly packed in a regular pattern and only vibrate in place, which makes solids rigid and incompressible. Everyday examples of solids include rocks, ice, and iron. Liquids have a fixed volume but no fixed shape, meaning they take the shape of their container. The particles in a liquid are close together but not in a fixed position, which allows them to slide past one another. This property makes liquids flow easily and assume the shape of their containers. Water, blood, and mercury are instances of liquids. Unlike solids, liquids have a surface that can assume a horizontal level in a container. Gases have neither a fixed shape nor a fixed volume. They will expand to fill any container they are placed in. This is because the particles in a gas are much farther apart than in solids or liquids, and they move around freely at high speeds. This high-speed movement and the significant space between particles make gases highly compressible. Examples of gases include oxygen, nitrogen, and carbon dioxide. In everyday life, we interact with gases through the air we breathe and the carbon dioxide released when we exhale. 7. What property is unique to gases compared to Questions with Multiple Choice solids and liquids? A) Fixed shape 1. What is a characteristic feature of solid B) High compressibility matter? C) Fixed volume A) Fixed volume but no fixed shape D) Incompressibility B) High compressibility 8. Why can't solids flow like liquids? C) Fixed shape and volume A) Because their particles move at high D) Assumes the shape of its container speeds 2. Which state of matter has particles that are B) Because their particles are in a fixed close together but not in fixed positions? position A) Solid C) Because they have no definite shape B) Liquid D) Because they are highly compressible C) Gas 9. Which of the following is not a property of D) Plasma liquids? 3. Why do gases fill the entire container they are A) Takes the shape of its container in? B) Flows easily A) Particles are tightly packed C) Particles are tightly packed in a regular B) Particles move at low speeds pattern C) Particles are far apart and move freely D) Has a fixed volume D) Particles have a fixed position 10. Which example fits a gas state of matter? 4. What makes liquids assume the shape of their A) Gold container? B) Olive oil A) Rigidity C) Nitrogen B) Incompressibility D) Sugar C) The ability to flow D) High-speed particle movement 5. Which of the following is an example of a solid? A) Water B) Oxygen C) Ice D) Alcohol 6. How are liquids different from solids in terms of volume? A) Liquids have no definite volume B) Liquids and solids both have no definite volume C) Liquids have a fixed volume D) Solids have no definite volume