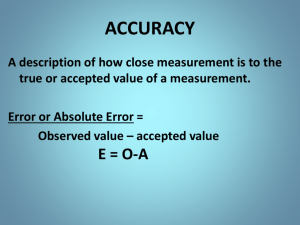
Lesson 2: MEASUREMENTS Prepared by: Mark C. Alderite, LPT Objectives: At the end of the lesson, the learners: 1. 2. 3. Explain the need for measurements; Describe how to carry out measurements of length, mass, and volume; and Differentiate between precision and accuracy 01 UNITS of MEASUREMENTS What do we mean by: 01 Length in measuring the height of a person; distances; the size of cloths 03 Mass in measuring the weight of a person; the amount of salt or sugar being bought 02 Volume in measuring the amount of a liquid (e.g. soft drinks) Time in measuring the duration of an event (e.g. to run through a distance) 04 Temperature in measuring the body temperature of a person or of the atmosphere. 05 SI units The International System of Units (SI) is the metric system used in science, industry, and medicine. Units of the SI System There are seven base units in the SI system: Kilogram (kg) – Mass Second (s) – Time Kelvin (K) – Temperature Ampere (A) – Electric Current Mole (mol) – Amount of substance Candela (cd) – Luminous intensity Meter (m) – Distance Joule (J) - Energy Imperial Systems (commonly used in US and UK) Gallons (gal), pint, ounces - Volume Feet (ft), Inches (inch), miles (mi) Length Pounds (lb), Ounces, stone – Mass Square feet and acres - Area SI UNIT PREFIXES Designation of multiples and subdivisions of unit by combining with the name of the unit. 02 Accuracy and Precision in Measurements Chemistry is an experimental science. As such measurements are important in conducting experiments, recording data and observations, and making conclusions. What is precision? Precision refers to how close each measurement is to one another. ● ● The precision is good if the individual measurements are close to the average. The precision is poor if the measurement have a wide deviation from the average value. What is accuracy? Accuracy refers to the closeness of the average value to the actual or true value, or most probable value. • • Precise measurements are most likely to be accurate. Measurements with high precision can be inaccurate. Remember that measurements could have errors. In scientific research, measurement error is the difference between an observed value and the true value of something. It’s also called observation error or experimental error. There are two main types of measurement error: • Random error (low in precision) is a chance difference between the observed and true values of something (e.g., a researcher misreading a weighing scale records an incorrect measurement). There are two main types of measurement error: • Systematic error (low accuracy) is a consistent or proportional difference between the observed and true values of something (e.g., a miscalibrated scale consistently registers weights as higher than they actually are). By recognizing the sources of error, you can reduce their impacts and record accurate and precise measurements. Gone unnoticed, these errors can lead to research biases like omitted variable bias or information bias. Significant Figures in Measurements and Calculations SIGNIFICANT FIGURES In chemistry, Significant figures are the digits of value which carry meaning towards the resolution of the measurement. They are also called significant figures in chemistry. Basic Law of Significant Figures 1. All non-zero digits are significant. 2. Zeroes between non-zero digits are significant. 3. A trailing zero or final zero in the decimal portion only are significant. Following are the significant figures rules that govern the determination of significant figures: 1. Those digits which are non-zero are significant. For example, in 6575 cm there are four significant figures and in 0.543 there are three significant figures. 2. If any zero precedes the non-zero digit then it is not significant. The preceding zero indicates the location of the decimal point, in 0.005 there is only one and the number 0.00232 has 3 figures. Following are the significant figures rules that govern the determination of significant figures: 3. If there is a zero between two non-zero digits then it is also a significant figure. For example; 4.5006 has five significant figures 4. Zeroes at the end or on the right side of the number are also significant. For example; 0.500 has three significant figures. 5. Counting the number of objects for example 5 bananas and 10 oranges have infinite figures as these are inexact numbers. Significant Figures Examples 4308 – 2. 40.05 – 3. 470,000 – 4. 4.00 – 5. 0.00500 – 1. 4 significant figures 4 significant figures 2 significant figures 3 significant figures 3 significant figures Assessment: How many significant figures are in each measurement? 1. 2. 3. 4. 5. 6. 246.32 107.854 100.3 0.678 1.008 0.00340 7. 8. 9. 10. 11. 12. 14.600 0.0001 700000 350.670 1.0000 320001 Thank you! Does anyone have any questions? CREDITS: This presentation template was created by Slidesgo, including icons by Flaticon, and infographics & images by Freepik Mark C. Alderite, LPT Science Teacher