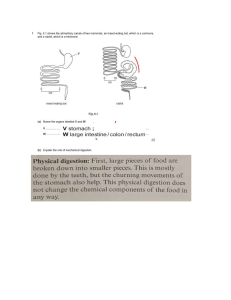
UNIVERSIDAD JAUME I NANOBIOSENSORS Vera Beltrán Pitarch, Luisa Hernández Górriz, Nacho Prada Barceló 0 Index 1. - Introduction. .................................................................................................................... 2 1.1. - Nanobiosensor structure........................................................................................... 3 1.2. - Nanobiosensor functioning. ...................................................................................... 4 1.3. - Selection and Optimization of Nanomaterials for Sensor Technology. ...................... 4 1.4. - Types of nanobiosensors. ........................................................................................ 8 1.5. - Applications of nanobiosensors. ............................................................................. 10 2. - Glucose nanobiosensors............................................................................................... 10 2.1 Brief History of Electrochemical glucose biosensors. ................................................. 11 2.2 Electrochemical biosensors. ...................................................................................... 12 2.3 Nanobiosensors......................................................................................................... 13 2.3.1 Nanoparticles. ..................................................................................................... 13 2.3.2 Nanocables, Nanofilms and nanofibers. .............................................................. 13 2.3.3 Carbon nanotubes............................................................................................... 14 2.3.4 Fluorescent polymeric nanosensors. ................................................................... 15 2.3.5 Quantum dots in glucose sensors. ...................................................................... 15 2.6 Conclusion................................................................................................................. 16 3. - Antibody-antigen recognition. ........................................................................................ 17 3.1. - Antibody structure. ................................................................................................. 17 3.2. - Detection of individual antibody-antigen recognition events by atomic force microscopy (AFM) ........................................................................................................... 18 3.3. Antibody´s recognition introduction ........................................................................... 19 3.4. Method ..................................................................................................................... 20 3.5. Results and discussion ............................................................................................. 21 3.6. Conclusions .............................................................................................................. 24 4. - Bibliography. ................................................................................................................. 26 1 1. - Introduction. A biosensor can be defined like an analytical device which incorporates a biologically active element with an appropriate physical transducer to generate a measurable signal proportional to the concentration of chemical species in any type of sample. It can detect a wide range of targets from small protein molecules to large pathogens. Some characteristics of biosensors are: ● Selectivity. Probably the most important feature of biosensors. Selectivity means that sensor detects a certain analyte and does not react to admixtures and contaminants. Antigen-antibody interaction has the highest selectivity, it is analytespecific. ● Accuracy. Is a characteristic of any scientific device that makes quantitative measurements. It is usually characterized in terms of the standard deviation of measurements. Signal error in measured concentration. Signal stability influences the accuracy of sensor. It is an important characteristic of a sensor that performs continuous monitoring. ● Sensitivity. It shows the minimal amount or concentration of analyte that can be detected. ● Working range. Is the range of analyte concentrations in which the sensor can operate. Working range of sensor should correlate with the range of possible concentrations analyte in the assay. ● Response time. Is the time required to analyze the assay. ● Regeneration time. The time required to return the sensor to working state after interaction with the sample. ● Number of cycles is the number of times the sensor can be operated. Degradation of biological material is inevitable and it needs to be replaced. Besides this, to be commercially successful, a biosensor has to meet the general requirements of commercial sensors that include specificity, accuracy, sensitivity, ease of use, reproducibility, near real-time assay, robustness, speed of response, running costs and life, and it has to be insensitivity to temperature and to electrical and other environmental interference. The number of false positive and false negative results of a biosensor should be very low, ideally zero, for it to be an acceptable practical device. A nanobiosensor is just a nanoscale biosensors that have exhibits rapid responses combined with very high sensitivities. 2 1.1. - Nanobiosensor structure. To work, the biosensor consists of three main parts: 1. Biological recognition element. It is a biologically derived material or biomimetic component that interacts (binds or recognizes) the analyte under study. It can be a tissue, microorganisms, organelles, cell receptors, enzymes, antibodies, nucleic acids, etc... 2. Transducer or detector element. Transforms the signal resulting from the interaction of the analyte with the biological element into another signal that can be more easily measured and quantified. It works in a physicochemical way: optical, piezoelectric, electrochemical, etc. 3. Signal processing electronics. Responsible for the display of the results in a userfriendly way. Figure 1. Nanobiosensor components. Figure 2. Elements of a biosensor. 3 1.2. - Nanobiosensor functioning. Broadly speaking, the nanobiosensor works as follows: In first place, bioreceptor recognizes the analyte. Then the biological material is immobilized and a contact is made between the immobilized biological material and the transducer. The analyte binds to the biological material to form a bound analyte which in turn produces the electronic response that can be measured. Following, the transducer converts the product linked changes into electrical signals which can be amplified and measured. And finally, the output from the transducer is amplified, processed and displayed. Figure 3. Nanobiosensor functioning. 1.3. - Selection and Optimization of Nanomaterials for Sensor Technology. There is a multitude, factors which govern or decide the use of a particular kind of nanomaterials for biosensing applications. These factors are the chief ingredients of their physical and chemical properties along with their energy sensitive and selective responses. Before exactly implementing or adding a nanomaterial for the sensing applications, we first focus on their desired manufacturing which is a part of experimental design known as “Nanofabrication.” The technique of nanofabrication targets two vital operations, namely, the manufacturing and design of nanoscale adhesive surfaces via the technology of integrated circuits and the engineering of nanomaterial surfaces through the process of micromachining. This technique, thus developed for biosensing, uses the variations of four basic processes, namely, photolithography, thin film etching/growth, surface etching strategies, and chemical bonding parameters. 4 Nanoscale electrodes which have come into picture as a result of lithography technique have enhanced the biosensing accuracy by providing much better and greater surface areas that in turn enable the immobilization to be achieved with greater precision. Glucose biosensors, making use of enzyme glucose oxidase, have been developed using these innovations. The strategies involving the use of active nanoparticles of platinum over the sheets of carbon nanotubes have significantly enhanced the immobilization of enzyme systems required for the detection of the analyte materials. These systems have significantly much wider applications to biosensing technology, enabling the detection of glucose from several sources other than blood. In a similar manner, couples of immunosensors have also been developed which involve coating of thin films over the sensing surface that enables faster and better detection of the corresponding analytes. Highly sensitive electrical and electromechanical properties are incorporated into several materials by engineering them with nanoelectromechanical systems (NEMS), which have enabled the generation of complex electrical, mechanical, fluidic, thermal, optical,and magnetic properties of the materials with sizes down to the nanometer level. NEMS technology has thus provided many materials with novel properties due to their nanoscale functionalization. NEMS and MEMS devices have enabled better and better sophisticated performance of the mechanical materials as the mechanical properties of a material are a critical function of its size. In addition, these devices have been coupled with biological systems and molecules to improve their bio adhesion characteristics and the response to a wide range of stimuli. With the implementation of NEMS and MEMS, surface forces like friction, adhesion, cohesive forces, and viscous drag forces can be controlled in a very precise manner that enables the best modeling of the biochemical interactions taking part in the biosensing technology. Another important factor considered while using nanomaterials for sensing application is the monitoring and optimization of their optical properties. The phenomena like surface plasmon resonance are very interesting and in particular expected from nanoparticles so as to maximize the sharp and precise scale optical response of the sensing materials with the incident light. The surface plasmon resonance effect is concerned with the excitation of particle surface with the ionic species and charged particles which create ions and result in excitation of the fluidic state of charged particles. This property is highly suitable in case of nanoparticles due to their unique optical properties which give them photonic character and excellent ability to be used as fluorophores. This phenomenon makes use of total internal reflection which takes place for angle of incidence reaching beyond a critical value. Here, 5 the reflection of light through a thin film of metallic nanoparticles coated over a surface is optimized by the corresponding adjustment of the critical angle of reflection. In case of nanomaterials, this phenomenon is highly logical and is especially named as localized surface plasmon resonance. Surface plasmon resonance effect is also dependent upon the refractive index of a medium and it is the most fundamental property governing the flow of light through a medium. Due to the phenomenon of surface plasmon resonance, a nanobiosensor is better equipped to detect the minutest interacting phenomenon, which enables a far greater and much reliable degree of estimation of biological interactions through a nanobiosensor in comparison with a biosensor. In this way, nanomaterials, irrespective of their nature, need to be optimized for their performance and effect as per the desired goal before being actually implemented for the biosensing purpose. Nanostructured semiconductor crystals can be efficiently used to improve the detection of neurological responses via coupling through the sensing molecule of biological nature. These can be coupled with peptide assembly of a range of nanomaterials so that efficient interaction can be generated by means of self-assembly and this also saves a lot of time that is being involved in the currently available technologies and methods. Moreover, these can rapidly detect the biological stimulus such as that of a DNA segment or a characteristic nucleotide sequence pertaining to proteins or even RNA. Moreover a key strategy into shaping-up of nanomaterials for desired applications involves the tuning and engineering of their surface by sophisticated inroads collectively termed as micromachining procedures. Factors like aspect ratios, functionalization with other materials and compatibility issues with respect to the material being analyzed for are highly critical for the use of nanomaterials in biosensing applications. There is a table of most common nanomaterials used in nanobiosensors: 6 Carbon nanotubes Carbon nanotubes were discovered in 1992 and they are cylindrical structures rolled up graphene sheets. Carbon nanotubes can be formed by one sheet with a diameter form 0,4 to 2nm or by a couple of sheets, with a diameter from 2 and 100nm. Figure 3: Dimensions of carbon nanotubes. These nanostructures have a good application in the construction of nanodevices, they permit the biocomponent to interact with a higher superficial area, providing higher conductivity and better electrical communication between surfaces and immobilized biocomponents. The combination of nanotubes with redox active enzymes has promoted the development of more reactive platforms in nanobiosensors. We can find various electrochemical biosensors systems, with an improvement in the catalytic signal compared with the one in the macrocarbon electrode. Nanoparticles The most widely used nanomaterial in industry overall to date, however, is the silver nanoparticle. These have also been harnessed as a simple electrochemical label in a highly sensitive amperimetric immunoassay intended for distributed diagnostics and as an inexpensive solution for immunoassays performed in developing countries. Most recently, nanostructured materials have been used to deliver label-free electrochemical immunoassays. Quantum dots Enzymes are essential in the human body, and the disorder of enzymatic activities has been associated with many different diseases and stages of disease. Luminescent semiconductor 7 nanocrystals, also known as quantum dots (QDs), have garnered great attention in molecular diagnostics. Owing to their superior optical properties, tunable and narrow emissions, stable brightness and long lifetime, QD-based enzyme activity measurement has demonstrated improved detection sensitivity, which is considered particularly valuable for early disease diagnosis. Recent studies have also shown that QD-based nanosensors are capable of probing multiple enzyme activities simultaneously. Nanowires Nanowire (NW)-based FETs are promising devices with potential applications ranging from health monitoring to drug discovery. In fact, these devices have demonstrated the ability to detect a variety of analytes such as particular DNA sequences, cancer biomarkers, and larger entities such as viruses. These sensor devices have also been used to monitor enzymatic activities and study the behavior of potential drug molecules. The detection of the analytes occurs with high specificity and sensitivity in reasonably short time. 1.4. - Types of nanobiosensors. Biosensors can be categorized according to the basic principles of signal transduction and biorecognition elements. In the general scheme of a biosensor (Figure 2), the biorecognition element responds to the target compound and the transducer converts the biological response to a detectable signal, which can be measured electrochemically, optically, acoustically, mechanically, calorimetrically, or electronically, and then correlated with the analyte concentration. Then we will go to introduce the most popular types of biosensing devices in use today. Micro-Biosensors The major progress in microsystem technologies for creating small, integrated and reliable microtransducers devices in combination with biological sensing elements has revolutionized the field of biosensors during the last decade. Such micro-biosensor systems raised the expectation to get a comprehensive insight into dynamic cellular metabolic events and subsequently a complete understanding of the metabolism of human biology. Currently, cancer can be detected by monitoring the concentration of certain antigens present in the bloodstream or other bodily fluids, or through tissue examinations. Correspondingly, diabetes is monitored by determining the glucose concentrations in the blood over time. However, despite their widespread clinical use, these techniques have a number of potential limitations. For example, a number of diagnostic devices have slow response times and are burdensome to patients. Furthermore, these assays are expensive and cost the health care industry billions of dollars every year. Therefore, there is a need to develop more efficient and reliable sensing and detection technologies. 8 Within this classification we can find: Electrochemical Biosensors, Potentiometric Biosensors, Amperometric Biosensors and Voltammetric Biosensors. Optical biosensors. Optical detection biosensors are the most diverse class of biosensors because they can be used for any different types of spectroscopy, such as absorption, fluorescence, phosphorescence, Raman, SERS, refraction, and dispersion spectrometry. In addition, these spectroscopic methods can all measure different properties, such as energy, polarization, amplitude, decay time, and/or phase. Amplitude is the most commonly measured as it can easily be correlated to the concentration of the analyte of interest [28]. In optical biosensors, the optical fibers allow detection of analytes on the basis of absorption, fluorescence or light scattering. Since they are non-electrical, optical biosensors have the advantages of lending themselves to in vivo applications and allowing multiple analytes to be detected by using different monitoring wavelengths. The versatility of fiber optics probes is due to their capacity to transmit signals that reports on changes in wavelength, wave propagation, time, intensity, distribution of the spectrum, or polarity of the light. In general, acquisition of the signal from these devices is accomplished through flexible cables, which can transmit light to the biological component. The most common optical biosensors are: Fluorescence-based Biosensors, Surface Plasmon Resonance Biosensors and Optical fiber based biosensors. Acoustic biosensors. Electroacoustic devices used in biosensors are based on the detection of a change of mass density, elastic, viscoelastic, electric, or dielectric properties of a membrane made of chemically interactive materials in contact with a piezoelectric material. Bulk acoustic wave (BAW) and surface acoustic wave (SAW) propagation transducers are commonly used. In the first, a crystal resonator, usually quartz, is connected to an amplifier to form an oscillator whose resonant frequency is a function of the properties of two membranes attached to it. The latter is based on the propagation of SAWs along a layer of a substrate covered by the membrane whose properties affect the propagation loss and phase velocity of the wave. SAWs are produced and measured by metal interdigital transducers deposited on the piezoelectric substrate. Even though SAW-based biosensor systems have been the focus of academic and industrial research for a number of years, most of these approaches only feature laboratory setups that are suitable for proof-of-principle evaluation and first experimental tests. For real commercial success, two crucial issues need to be solved: an appropriate production process is required, as is an applicable handling process for future SAW based biosensors. Since 1959, the most used acoustic device for sensor has been The Quartz Crystal Microbalance (QCM). 9 1.5. - Applications of nanobiosensors. Biosensors have been intensively studied and extensively utilized in various applications such as: ● DNA sensors: Genetic monitoring, disease. ● Immunosensors: HIV, Hepatitis, other viral diseas, drug testing, environmental monitoring… ● Cell-based sensors: functional sensors, drug testing… ● Point-of-care sensors: blood, urine, electrolytes, gases, steroids, drugs, hormones, proteins, … ● Bacteria sensors: food industry, medicine, environmental, … ● Enzyme sensors: diabetics, drug testing, ... In this document we will focus on nanobiosensors dedicated to the detection of glucose in the blood (enzyme sensor) and antigen-antibody recognition. 2. - Glucose nanobiosensors. One of the most common application of the nanobiosensors is used to detect the glucose levels in the blood. Diabetes is a health problem that affects millions of people all over the world and part of the treatment involves monitoring the glucose levels in blood with the nanobiosensors and the enzyme glucose oxidase, so that there can be action taken if these levels go very high. Glucose oxidase is a small and stable enzyme that oxidizes the glucose into gluconolactone, converting oxygen into hydrogen peroxide, which is sensed by a platinum electrode. The hydrogen peroxide is a toxic compound that can be used to kill bacteria. The more peroxide is formed; the stronger the signal is at the electrode. The glucose oxidase converts something that is difficult to measure, the glucose, into something easy to measure, the hydrogen peroxide. In the last decades it has been a very fast development in the investigation of the nanobiosensors for controlling the levels of the glucose in blood. The nanobiosensors nowadays are one of the most important achievements in nanotechnology, due to the fact that their biocomponents increase sensibility, stability, miniaturization and exchange of microfluidics with different parts of the body. In particular, the investigation of nanobiosensors for monitoring the glucose levels has promoted also the design of new nanomaterials and the integration of nanostructured areas or nanomaterials useful for developing the construction of biocompatible devices. Glucose is the main source of energy for the cells, it is transported to the cells through the insulin in blood. The human body regulates the levels of glucose in the blood in a concentration of 4-8mM (70-120mgdL-1). Diabetes is a metabolic disease that produces 10 anormal levels of glucose in blood and consequently inflammation and apoptosis events in cells. There are very important problems associated to this disease, like problems in the retina, circulatory system or kidneys. It is estimated that there will be an increase of 69% of diabetes patients in the developing countries and a 20% in the developed countries. In order to control the glucose levels in blood and reduce complications of hyper and hypoglycemia, the scientific community has devoted important resources on the development of intelligent diagnostic tools to fight against diabetes. Patients need to control the glucose levels in blood very frequently with a painful measurement and also huge variations in the monitoring, consequently the increasing demand of new technologies to satisfy a continuous and noninvasive monitoring of glucose with high precision and low cost. Traditional monitoring of blood glucose uses discrete blood sampling time points during the course of a day, however continuous monitoring allows the patient to intercede and avoid complications, highlighting the advantages of an increased frequency of measurement. Another advantage is the estimation of future blood glucose levels and the possibility to monitor without the patient intervention. On the other hand it has some disadvantages as all the provided devices are implanted sensors which have a maximum useful lifetime of several days to a week and as furthermore they have a time lag in measurements taken during periods of rapid concentration changes, estimated from several minutes to nearly 30 minutes. Finally, current sensors are expensive and not always covered by health insurance plans so the technology has not been widely adopted. 2.1 Brief History of Electrochemical glucose biosensors. The history of glucose enzyme electrodes began in 1962 with the development of the first device by Clark and Lyons of the Cincinnati Children’s Hospital. Their first glucose enzyme electrode depended on a thin film of GOx (glucose oxidase) catched by an oxygen electrode with a semipermeable dialysis membrane. The oxygen consumed by the enzyme-catalyzed reaction was monitorized. A negative potential was applied to the platinum cathode for a reductive detection of the oxygen consumption. The entire field of biosensors can trace its origin to this original glucose enzyme electrode. In 1973, Guilbault and Lubrano described an enzyme electrode for the measurement of blood glucose based on amperometric monitoring of the hydrogen peroxide product. The resulting biosensor offered good accuracy and precision. Use of electron acceptors for replacing oxygen in GOx-based blood glucose measurements was demonstrated in 1974. In 1974 was proposed the ex-vivo monitoring of blood glucose and in 1982 was demonstrated the in-vivo glucose monitoring. During the decade of the 1980 considerable efforts were made focused on the development of mediator-based ‘second-generation’ glucose biosensors, introduction of commercial 11 screen-printed strips for selfmonitoring of blood glucose, and use of modified electrodes and tailored membranes for enhancing sensor performance. In the 1990s, there was extensive activity directed toward the establishment of electrical communication between the redox center of GOx and the electrode surface. 2.2 Electrochemical biosensors. The biosensors combine the selectivity of the enzymes with the simplicity of the amperometric transductors. Figure 1 shows the working process of the device. The analyte spreads through the solution and throw the membrane until it arrives at the active center of the enzyme where it reacts creating the product, generally with redox properties. This is oxidized on the electrode and creates a new product spread onto the solution again. Figure 1: Glucose detector working process. We can find three different types of amperometric biosensors with different methods to transfer electrons between the enzyme and the amperometric transduction. Figure 2: Three generations of biosensors based on oxidoreductases. 12 2.3 Nanobiosensors. Nanobiosensors are a big advance in order to measure the glucose levels in blood, they have numerous advantages that can contribute in the improvement of the attention to the diabetic patients. Nanobiosensors increase the sensibility in the quantification limits and provide a better analysis and the use of nanomaterials with biocomponents improves the stability and specificity of the system detection and the reliability. Nanotechnology allows the miniaturization and integration of biocomponents in complex nanobiosensors, capable to monitor continuously the glucose with implantable devices like the lab-on -chip for the rapid detection and low cost. These characteristics have motivated the investigators to explore alternative strategies based on the different nanotechnologies of nanomaterials or nanostructures, to develop the optical or electrochemical biosensors. Numerous kinds of nanomaterials have been used to develop biosensors, related to particles, nanotubes, nanocables, nanowires, nanofibers, nanocompounds, nanofilms, nanopolímers and nanoplates. These materials are capable of improving the performance of the detection systems due to the physic, chemical, mechanical, optical and magnetic characteristics. The development of nanotechnology has contributed to the efforts to make real the use of nanomaterials to detect glucose, in a faster response time, operativity stability, selectivity and easy measurement. 2.3.1 Nanoparticles. Metallic nanoparticles (NP) have been used in the electrochemical biosensors showing great advantages in the sensibility of the concentration limits. Gold nanoparticles (AUNPs) are used to fix the biocomponents in the established platforms. They have excellent biocompatibility between proteins and gold and also good electroanalytical applications. It has been demonstrated that the immobilization of the enzyme glucose oxidase in the gold nanoparticles provides better stability in the biosensors for a long period of time and also improves the analytical performance of the biosensors. Nanoparticles of iron oxide (Fe3O4) have also good properties to immobilize the biocomponents, due to the biocompatibility, paramagnetic properties and low toxicity. 2.3.2 Nanocables, Nanofilms and nanofibers. Nanocables, nanofilms and nanofibers have exclusive characteristics for the electrons transfer and superficial area. These materials can be incorporated in high density molds providing a higher superficial area of high current density and producing an increase on the transference of electrons and a better sensibility. Biosensors based in nanocables were developed in 2010 by immobilization of glucose oxidase with nanocables what made an increase on the enzyme absorption and electrons 13 transfer with the area. It produced high sensibility, low detection limits, fast response and good sensor stability. Nanofibers together with other polymeric conductor nanostructures have been extensively used like materials for the biosensors. They have special properties and have been used for different applications since the electrodes, capacitors and molecular conductor wires development for the immobilization of the biologic material in the sensors. Nanoparticles of polietilendioxitofen (PEDOT) are used for the deposition of aladio and glucose oxidase nanoparticles and nanofibers of Co3O4 for the construction of a no enzymatic sensor that detects glucose and shows high sensibility, reproducibility and selectivity. 2.3.3 Carbon nanotubes. The incorporation of nanomaterials in the sensors, as we have described before, provides a variety of advantages including increased surface, more efficient electron transfer from enzyme to electrode and the ability to include additional catalytic steps. The most common modification in the enzymatic electrode detection of glucose is the carbon nanotube incorporation. Another approach is to modify the nanotubes with an electrochemical mediator such as ferrocene to improve the electron transfer between the enzyme and the electrode. Combining nanotubes with additional nanomaterials (silver, platinum, gold nanoparticles, etc.) improves aspects such as catalytic activity. Nanostructured polymers can improve the development of glucose sensors. Hollow spheres of conductive polymer can be used to transfer electrons from GOx to the electrode; Conductive polymer electrodes can be used in a method similar to other nanostructured surfaces, where GOx is immobilized directly in the modified electrode. The use of polymers introduces the operation at varying potentials. However, the sensors based on biological recognition have several disadvantages including a poor stability compared with the nonbiological systems. As a result of this limitation many research groups have focused on the development of glucose detection assays that do not rely on a protein for recognition and, as a result, could have longer storage lifetimes. The most developed research area in nonenzymatic glucose sensors is detection of glucose oxidation directly at an electrode. Glucose detection has been demonstrated using copper and copper oxide nanowires, porous films as well as nanoflowers and nanorods. Several of the direct glucose oxidation sensors perform in biological samples; however they will not see much utility in clinical settings without significant work to improve their ability to work in undiluted samples, those obtained routinely by patients. 14 2.3.4 Fluorescent polymeric nanosensors. For in-vivo continuous monitoring, fluorescence based sensors offer the advantage to optically interrogate the sensors through the skin rather than having an electrode system implanted. This approach often involves a “smart tatoo” for the patient, as sensors would be implanted into the skin of the patient. That would be only temporary and would need to be replaced on weeks or months. These sensors would change fluorescence properties in response to blood glucose, and this change could be read out using optical interrogation through the skin. This method would eliminate or reduce the need for patients to take blood samples while allowing data to be collected in a more continuous manner. A variety of nanosensor technologies have been developed using fluorescence signals. 2.3.5 Quantum dots in glucose sensors. Semiconductor quantum dots (QDs) have excellent optical properties to use in sensors such as narrow fluorescence peaks and minimal photobleaching. However they do not interact with glucose, and consequently have no inherent recognition ability and must be coupled to a recognition element for successful implementation. To fabricate sensing systems it is used the cadmium telluride QDs with the GOx. They allow rapid optical detection of glucose 2.4. Home Testing of Blood Glucose Since blood glucose home testing devices are used daily to diagnose life-threatening events they must be of extremely high quality. The majority of personal blood glucose monitors rely on disposable screen-printed enzyme electrode test strips. Each strip contains the printed working and reference electrodes, with the working one coated with the necessary reagents and membranes. Despite these remarkable technological advances, home testing of blood glucose often suffers from low and irregular testing frequency or inadequate interpretation of the results by the patient and requires compliance by patients. More integrated devices offering multifunctional capability and convenient monitoring of changes in the glucose level are expected in the near future. Although self-testing is considered a major advance in glucose monitoring, it is limited by the number of tests per day it permits and results in poor approximation of blood glucose variations. Tighter glycemic control, through continuous monitoring, is desired for detecting changes in the glucose level and to activate an alarm in cases of hypo- and hyperglycemia. Continuous glucose monitoring provides maximal information about changing blood glucose levels throughout the day, including the magnitude, duration, and frequency of that fluctuations, and provides the opportunity of making fast and optimal therapeutic interventions. 15 The ideal sensor would be one that provides a reliable real-time continuous monitoring of all blood glucose variations throughout the day with high selectivity and speed over extended periods under hard conditions. An undesirable interaction between the surface of the implanted device and biological medium cause deterioration of the sensor performance and it is a barrier to the development of reliable in-vivo glucose probes. In blood, a complication arises from surface fouling of the electrode by proteins and coagulation composites and the risk of thromboembolism consequently most glucose biosensors don’t have the biocompatibility necessary for reliable prolonged operation in blood. Accordingly, the majority of the sensors being developed for continuous glucose monitoring do not measure blood glucose directly. Alternative locations, particularly the subcutaneous tissue, have received growing attention. It is minimally invasive, and its glucose level reflects the blood glucose concentration. However, such subcutaneous implantation generates a wound location that experiences an intense local inflammatory reaction. Although major advances have been made and several short-term in-vivo glucose sensors are approaching the commercial stage, major efforts are required before a reliable long-term minimally invasive or noninvasive sensing becomes a reality. 2.5. Subcutaneous Monitoring Most of the recent attention regarding real-time in-vivo monitoring has been given to the development of subcutaneously implantable needle-type electrodes. Such devices track blood glucose levels by measuring the glucose concentration in the interstitial fluid of the subcutaneous tissue. Subcutaneously implantable devices are commonly designed to operate for a few days, after which they are replaced by the patient. They are commonly inserted into the subcutaneous tissue in the abdomen or upper arm. Success in this direction has reached the level of short-term human implantation; continuously functioning devices, possessing adequate stability, are expected in the very near future. Such devices would enable a swift and appropriate corrective action through use of a closed-loop insulin delivery system, i.e., an artificial pancreas. Computer algorithms correcting for the transient difference (short time lag) between blood and tissue glucose concentrations have been developed. Subcutaneously implantable glucose sensors have moved from the purely experimental stage to commercially available products. 2.6 Conclusion. The success of glucose blood meters has stimulated considerable interest in in-vitro and invivo devices for monitoring other physiologically important compounds and new materials and concepts, developed originally for improving glucose biosensors, now benefit a wide range of sensing applications. 16 Fundamental progress has been made in this area to improve the reliability of glucose measuring devices but despite the impressive progress in the development of glucose biosensors, the promise of tight diabetes management has not been accomplished, and there are still many challenges related to the achievement of a highly stable and reliable continuous monitoring. In order to achieve this goal the future work should emphasize in testing with realistic, clinical samples and the cost and effort of the new sensor approaches should provide also improvements in the patient quality life but minimal additional new cost. Nanoscale sensors have the potential to improve continuous glucose monitoring capabilities and improve patient quality of life, allowing more long-term monitoring and reaching the goal of closed-loop artificial pancreas. The closed-loop pancreas will eliminate the need for the patient to perform the steps to inject the insulin, as it would perform all the tasks, similar to a natural pancreas. 3. - Antibody-antigen recognition. Antigen-antibody interaction, or antigen-antibody reaction, is a specific chemical interaction between antibodies produced by B cells of the white blood cellsand antigens during immune reaction. It is the fundamental reaction in the body by which the body is protected from complex foreign molecules, such as pathogens and their chemical toxins. In the blood, the antigens are specifically and with high affinity bound by antibodies to form an antigenantibody complex. The immune complex is then transported to cellular systems where it can be destroyed or deactivated. There are several types of antibodies and antigens, and each antibody is capable of binding only to a specific antigen. The specificity of the binding is due to specific chemical constitution of each antibody. The antigenic determinant or epitope is recognized by the paratope of antibody, situated at the variable region of the polypeptide chain. The variable region in turn has hypervariable regions which are unique amino acid sequences in each antibody. Antigens are bound to antibodies through weak and noncovalent bonds such as electrostatic interactions, hydrogen bonds, Van der Waals forces, and hydrophobic interactions. The principles of specificity and cross-reactivity of the antigen-antibody interaction are useful in clinical laboratory for diagnositic purposes. One basic application is determination of ABO blood group. It is also used as a molecular technique for infection with different pathogens, such as HIV, microbes, and helminth parasites. 3.1. - Antibody structure. In an antibody, the FAB (fragment, antigen-binding) region terminates into an aminoterminal end of both the light and heavy chains of the immunoglobulin polypeptide. This region called V (variable) domain is composed of amnio acid sequences that define each type of antibody and their binding affinity to an antigen. The combined sequence of variable light chain (VL) and variable heavy chain (VH) creates three hypervariable regions (HV1, HV2, 17 and HV3). In VL these are roughly from residues 28 to 35, from 49 to 59, and from 92 to 103, respectively. HV3 is the most variable part. Thus these regions are the paratope, the binding site of antigen. The rest of the V region between the hypervariable regions are called framework regions. Each V domain has four framework domains, namely FR1, FR2, FR3, and FR4. 3.2. - Detection of individual antibody-antigen recognition events by atomic force microscopy (AFM) A methodology has been developed for the study of molecular recognition at the level of single events and for the localization of sites on biosurfaces, in combining forcé microscopy with molecular recognition by specific ligands. For this goal, a sensor was designed by covalently linking an antibody (anti-human serum albumin, polyclonal) via a flexible spacer to the tip of a force microscope. This sensor permitted detection of single antibody-antigen recognition events by force signals of unique shape with an unbinding force of 244 + 22 pN. Analysis revealed that observed unbinding forces originate from the dissociation of individual Fab fragments from a human serum albumin molecule. The two Fab fragments of the antibody were found to bind independently and with equal probability. The flexible linkage provided the antibody with a 6-nm dynamical reach for binding, rendering binding probability high, 0.5 for encounter times of 60 ms. This permitted fast and reliable detection of antigenic sites during lateral scans with a positional accuracy of 1.5 nm. It is indicated that this methodology has promise for characterizing rate constants and kinetics of molecular recognition complexes and for molecular mapping of biosurfaces such as membranes. 18 3.3. Antibody´s recognition introduction The invention of Scanning Force Microscopy (SFM) and its modification to optical detection of forces has opened the exciting perspective of imaging the surface of living biological specimens. The additional potential of SFM for the study of molecular recognition, using a measuring tip with ligands bound, has recently gained much attention. The idea is to detect and study the binding of ligands on tips to surface-bound receptors by applying an increasing force to the complex that reduces its lifetime until it dissociates at a measurable unbinding force. So far, interaction forces were reported for the ligand-receptor pair biotin-avidin and for complementary DNA nucleotides. For these studies, SFM tips were covered with immobilized ligands. This strategy failed for antibody-antigen recognition (1), and the failure was attributed to the lack of molecular mobility and to unspecific tip-probe adhesion forces, obscuring specific interactions. Apart from detection and study of single recognition events, the concept of using SFM tips with ligands ("sensors") has further perspectives: (i) (ii) (iii) for localizing individual recognition sites. for imaging their distribution at surfaces. for combining recognition imaging by ligands with structural imaging by the tip as a method for molecular mapping of the topography of biosurfaces. Advances toward this goal are expected from the realization of an appropriately designed sensor. The ideal sensor configuration appears to be a single ligand on a tip that is free to orient and move for unconditioned recognition during surface imaging by the tip. We approached this configuration by covalently coupling ligands to tips via a long flexible spacer 19 molecule at a sufficiently low ligand concentration so that about one ligand is expected to have access to surface-bound receptors. We are going to use an antibody as the sensor molecule trying to demonstrate the suitability of this sensor design for detection and characterization of single antibodyantigen recognition events and for the localization of antigenic sites. Perspectives of the method are discussed. 3.4. Method Preparation of Sensor and Probe. A newly synthesized 8nm long polyethylene glycol (PEG) derivative with an aminereactive end and a thiol-reactive end was used as spacer for covalent linkage of the antibody to silicon nitride tips and of the antigen to mica surfaces. Tips were cleaned by a standard procedure for 10 min in chloroform and for 30 min in H2SO4/H202, 70:30 (vol/vol), and then extensively rinsed with deionized water. Surface-bound water was removed by drying freshly cleaved mica and cleaned tips in an oven for 2 hours at 180°C. For functionalization with amine-containing groups, tips and mica sheets were then immediately esterified in a solution of ethanolamine chloride in dimethyl sulfoxide, overnight at 100°C with 0.3-nm molecular sieve beads while applying an aspirator vacuum and trapping H20 in a CaCl2 tower. Binding of PEG to amine-containing substrates and consecutive coupling of antibody and antigen to the thiol-reactive end of the spacer was done as described. Determination of Surface Density: Sensitive high-resolution fluorescence imaging with accurate calibration was employed for the determination of the surface density of HSA and antibody, which had been fluorescence-labeled prior to surface linkage. Probes with high HSA densities showed considerable clustering in fluorescence images. Force Microscopy. Cantilevers of sensors had spring constants between 0.11 and 0.27 N/m. Briefly, a silicon cantilever with a spring constant of kr = 0.18 ± 0.02 N/m was taken as reference lever. The silicon nitride cantilevers used in the study were calibrated by comparing their repulsive force-distance slopes on a solid support with slopes found in contact with the reference lever. All silicon nitride cantilevers were calibrated with the same reference lever and calibrations were carried out in buffer. 20 Adhesion forces between sensor tip and probe were generally absent. For applications of the sensor to biosurfaces, sensors showed no adhesion to a cell membrane (mast cell) or to a lipid membrane (dimyristoylphosphatidylcholine) on mica in aqueous solution with 150 mM NaCl. 3.5. Results and discussion For a sensor molecule on the SFM tip, we chose a polyclonal anti-HSA antibody. The antibody was covalently linked to the tip. The density of antibodies on tips was adjusted to best meet the expectation that only one antibody may interact with the probe. More specifically, the SFM tips carried many antibodies. Their surface density was, however, chosen sufficiently low so that, on the average, only about one of the flexibly linked antibodies is expected to be bound to the tip end that will reach to HSA molecules on the probe surface. Forces between sensors and probes with high HSA surface density were monitored during force-distance cycles (figure A) by moving the probe continuously up ("trace") and down ("retrace") at constant lateral positions. It presents evidence that the sensor permits detection of single antibody-antigen binding events. (A) Effect of antibody-antigen binding on the force between tip and probe, illustrated for a typical record of a force-distance cycle. Binding of the antibody to the antigen during approach connects tip to probe. This causes an extra force signal of particular shape during tip retraction ("retrace"), reflecting extensión of the flexible connection to its full length. The force increases until unbinding occurs at the unbinding force. The length of the connection at the moment of unbinding was determined by subtracting tip deflection from the distance between contact and unbinding. Retraces of force-distance cycles showed attractive force signals of unique shape, interpreted to reflect antibody-antigen recognition. The attractive force develops nonlinearly with a significant delay as expected from stretching the long and flexible tipprobe connection after antibodyantigen association. The connection sustains the increasing force until the complex dissociates at a characteristic "unbinding force,". Such signals were repeatedly observed in consecutive force-distance cycles. Typically, 700 cycles were recorded at one fixed lateral position for which the 50 retraces shown in figure B are 21 representative. Twenty five of the retraces show one or two unbinding events. In the other cycles, there was no event detectable and retraces were identical to traces. (B) Force records of consecutive force-distance cycles. Unbinding events, shown here as upward signals, occurred in 25 out of the 50 records (only retraces are shown). One experiment is shown in figure C. The binding probability, Pb, which is the probability for observing an unbinding event in a forcedistance cycle, was followed before and after addition of HSA and after its removal (wash). The high initial value of Pb is immediately reduced to about 10% upon addition of free HSA and fully recovered about 27 min after perfusion with buffer. The few events detected after addition of free HSA are attributed to adsorption ofHSA molecules from solution to the probe surface. In additional controls using tips and probes with the PEG spacer bound but not conjugated with antibody and HSA, retraces were generally devoid of unspecific sensorprobe adhesion forces (retraces and traces were identical). Small adhesion forces were resolvable in less than 1% of the retraces that were easily discernible from unbinding events due to their clear separation from the delayed occurrence of unbinding. From controls and from observing unique signals of expected shape in the absence of adhesion forces, it appears safe to conclude that the signals are specifically due to antibody-antigen recognition. The ability of the antibody to bind, tested in more than 8000 force-distance cycles with four sensors, was found not to deteriorate, even after 2 month of storage in buffer. The force signal for unbinding contains information about antibody-antigen recognition at the level of single molecule interaction. (C) Time profile of antibody-antigen unbinding events for block by free HSA and for its recovery upon HSA removal. The probability per force-distance cycle for antibody binding to surface-bound HSA was Pb = 0.49 (0-30 min). Injection of 0.15 tpM free HSA resulted in a sudden and effective decrease of Pb to 0.06. After 30 min, the probe was washed for 1 min 22 with buffer after which unbinding events fully recovered within 27 min. Bars indicate times of continuous recordings of force-distance cycles at 1 Hz and their heights show the mean Pb values observed. In the figure 2 shows unbinding force and unbinding length distributions, determined from 201 consecutive forcedistance cycles at one position on a probe at high HSA density This unitary force is attributed to the recognition of single HSA molecules by one of the two binding sites of the antibody. The distribution of sensor length lu in figure 2B extends to values up to 30 nm. Irrespective of the apparent difference in sensor configuration during unbinding, the two Fab fragments showed virtually identical unbinding force distributions (Figure 2A). These findings indicate that the configuration of the molecular link and its momentary length during unbinding does not influence the unbinding force, attributed to the flexibility introduced by the spacer molecule. The described analysis was used to select sensors with effectively one antibody having excess to surface-bound antigens, selected by criterion of no more than two events per retrace. This applied to about 30% of the sensors in preparations at appropriate antibody surface density. For the other sensors up to six events were found in force-distance cycles, indicating that up to three antibodies were able to bind to HSA on the surface. The flexibly linked antibody was not found to be entrapped between tip and probe since the points of tip-probe contact in force-distance cycles were as expected for a plain surface. This also indicates that the imaging capability of the tip is not significantly perturbed by the chemical treatment for antibody linkage. This opens the perspective for a microscopy capable of simultaneously imaging surface topography and the distribution of recognition sites or of mapping the molecular topography of biosurfaces. While the sensor appears apt for such uses, realization will require software extension of measuring modes to area scans during force-distance cycles at an appropriate feedback control that is currently being developed.t Potentials for Studying Kinetics of Antibody-Antigen Interaction. From the dynamical reach reff and the vertical scan velocity, it is possible to estimate the antibodyantigen association rate constant kass. 23 This confirms the conclusion that the antibody is quite free to move and orient for binding within the constraints set by its dynamic reach, which compares with the length of the PEG spacer. The recovery time from block by free HSA of 1500 s is a direct measure for the lifetime of the antibody-antigecomplex in the absence of force or for its dissociation rate constant. Figure 3. Localization of antigen sites by a scanning tip-antibody sensor. (A) Histogram of unbinding events for the sensor passing one HSA molecule. The probe was laterally moved at 0.6 nm/s during force cycles at 3 Hz with a 100-nm amplitude. The number of events was sampled every 2.6 nm, corresponding to 13 cycles. The recognition profile represents 23 unbinding events in total. Mean distance between HSA molecules was 100 nm, as determined by fluorescence microscopy. Statistical analysis showed that the peak position reflects the true mean of the distribution within an uncertainty of 1.5 nm. (B) Overlay of binding profiles as seen inA. The distribution contains the data from six profiles, normalized to the average binding probability of 0.38, at maximum. It has a width of 6 nm, determined from the standard deviation of the Gaussian fit shown. All events occurred singly in force-distance cycles, indicating recognition of single HSA molecules. The mean fu value was 270 pN for the chosen cycle rate of 3 Hz and cantilever spring constant of 0.22 N/m. 3.6. Conclusions Flexible linkage of an antibody to an SFM tip has allowed thedetection of single recognition events between an antibody and an antigen. Analysis of force profiles revealed insight into the process of antibody-antigen binding and unbinding at the level of single molecular events, unconstrained by the linkage used. Antigenic sites were reliably detected during lateral scans. This was rendered possible by realization of a sensor design with effectively one antibody covalently coupled to the tip via a sufficiently long flexible spacer molecule. The functional groups of the spacer used are applicable to coupling of ligands and proteins in general, which provides the method with a broad perspective in the study of molecular recognition. With the possibility of combining molecular recognition by ligands with structural resolution by the tip, a first tool comes into sight for molecular mapping the topography of biosurfaces. 24 Schematic diagram for metal oxide nanostructure-based biosensing devices. The generation of output potential for the fabricated sensor device could result from the reaction mechanism, which creates a charged environment around the working electrode, and the resulting potential difference can be measured using a pH meter. It has been shown that CuO itself exhibits catalytic properties that can improve the efficiency of cholesterol oxidase and create a rapid and direct electron transfer rate between the active sites of cholesterol oxidase and its own surface. Moreover, the fabricated cholesterol biosensor using a bundle of CuO nanowires was found to be highly reproducible, repeatable, stable and selective. TEM images of nanowire bundles; (d) calibration curve of cholesterol biosensor with detection limit; and (e) response time of the cholesterol biosensor in the different cholesterol concentrations [98]. 25 4. - Bibliography. • NANOBIOSENSORS. Nano Science & Technology http://es.slideshare.net/tabirsir/nanobiosensors-5734391 Consortium. Disponible en: • NANO-TECHNOLOGY. DEVELOPMENT OF NANO-BIOSENSORS. Thompson Research Group. The University of Southern California. Disponible en: http://met.usc.edu/projects/nano.php • Florinel-Gabriel Banica. CHEMICAL SENSORS AND BIOSENSORS: FUNDAMENTALS AND APPLICATIONS. • Ahmed Touhami. BIOSENSORS AND NANOBIOSENSORS: DESIGN AND APPLICATIONS. Physics & Astronomy Department, University of Texas at Brownsville • RECENT TRENDS IN NANOBIOSENSORS AND THEIR APPLICATIONS - A REVIEW. Department of Physics, Sree Sastha Institute of Engineering and Technology, Chembarambakkam, Chennai-600 123, India. Department of Biotechnology, Sree Sastha Institute of Engineering and Technology, Chembarambakkam, Chennai-600 123, India. • NANOSENSORS WITH APPLICATIONS. Prathamesh V. Kolekar Dept. Instrumentation Engg. Vishwakarma Institute of Technology, Pune. • THE GRAMICIDIN-BASED BIOSENSOR: A FUNCTIONING NANO-MACHINE. Novartis Found Symp. 1999;225:231-49; discussion 249-54. • NANOBIOSENSORS: CONCEPTS AND VARIATIONS. Parth Malik,1 Varun Katyal,2 Vibhuti Malik,3 Archana Asatkar,4 Gajendra Inwati,1 and Tapan K. Mukherjee5. ISRN Nanomaterials Volume 2013 (2013), Article ID 327435 • QUANTUM DOT-BASED NANOSENSORS FOR DIAGNOSIS VIA ENZYME ACTIVITY MEASUREMENT. Expert Rev Mol Diagn. 2013 May. Knudsen BR1, Jepsen ML, Ho YP. • REAL-TIME, LABEL-FREE DETECTION OF BIOLOGICAL ENTITIES USING NANOWIRE-BASED FETS. Curreli, M. ; Dept. of Chem., Univ. of Southern California, Los Angeles, CA ; Rui Zhang ; Ishikawa, F.N. ; Chang, Hsiao-Kang. • Peter hinterdorfer, werner baumgartner, hermann j. Gruber, kurt schilcher, and hansgeorg schindler. Institute for biophysics, university of linz, a-4040 linz, Austria. 26