Borgnakke and Sonntag
12.1
The slope dP/dT of the vaporization line is finite as you approach the critical
point, yet hfg and vfg both approach zero. How can that be?
The slope is
hfg
dP
dT = Tv
sat
fg
Recall the math problem what is the limit of f(x)/g(x) when x goes
towards a point where both functions f and g goes towards zero. A finite limit for
the ratio is obtained if both first derivatives are different from zero so we have
dP/dT → [dhfg /dT] / d(Tvfg)/dT as T → Tc
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.2
In view of Clapeyron’s equation and Fig. 2.4, is there something special about ice
I versus the other forms of ice?
Yes. The slope of the phase boundary dP/dT is negative for ice I to liquid
whereas it is positive for all the other ice to liquid interphases. This also means
that these other forms of ice are all heavier than liquid water. The pressure must
be more than 200 MPa ≈ 2000 atm so even the deepest ocean cannot reach that
pressure (recall about 1 atm per 10 meters down).
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.3
If we take a derivative as (∂P/∂T)v in the two-phase region, see Figs. 2.7 and 2.8,
does it matter what v is? How about T?
In the two-phase region, P is a function only of T, and not dependent on v.
The slope is the same at a given T regardless of v. The slope becomes
higher with higher T and generally is the highest near the critical point.
P
L
C.P.
V
T
S
v
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.4
Sketch on a P-T diagram how a constant v line behaves in the compressed liquid
region, the two-phase L-V region and the superheated vapor region?
P
vsmall> vc
v < v Cr.P.
c
S
L
P
v < vc
V
L
v
medium
v
large
T
C.P.
v > vc
vmedium
S
V
T
v
large
v
The figures are for water where liquid is denser than solid.
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.5
If I raise the pressure in an isothermal process does h go up or down for a liquid
or solid? What do you need to know if it is a gas phase?
Eq. 12.25:
∂h
∂v
(∂P)T = v – T (∂T)P = v[1 - TαP]
Liquid or solid, αP is very small, h increases with P ;
For a gas, we need to know the equation of state.
12.6
The equation of state in Example 12.3 was used as explicit in v. Is it explicit in P?
Yes, the equation can be written explicitly in P.
RT
P=
C
v+ 3
T
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.7
Over what range of states are the various coefficients in Section 12.5 most useful?
For solids or liquids, where the coefficients are essentially constant over a
wide range of P’s and T’s.
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.8
For a liquid or a solid is v more sensitive to T or P? How about an ideal gas?
For a liquid or solid, v is much more sensitive to T than P.
For an ideal gas, v = RT/P , varies directly with T, inversely with P.
12.9
Most equations of state are developed to cover which range of states?
Most equations of state are developed to cover the gaseous phase, from
low to moderate densities. Many cover high-density regions as well, including
the compressed liquid region. To cover a wider region the EOS must be more
complex and usually has many terms so it is only useful on a computer.
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.10
Is an equation of state valid in the two-phase regions?
No. In a two-phase region, P depends only on T. There is a discontinuity
at each phase boundary. It is actually difficult to determine the phase boundary
from the EOS.
12.11
As P → 0, the specific volume v → ∞. For P → ∞, does v → 0?
At very low P, the substance will be essentially an ideal gas, Pv = RT, so
that v becomes very large. However at very high P, the substance eventually must
become a solid, which cannot be compressed to a volume approaching zero.
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.12
Must an equation of state satisfy the two conditions in Eqs. 12.49 and 12.50?
It has been observed from experimental measurements that substances do
behave in that manner. If an equation of state is to be accurate in the near-critical
region, it would have to satisfy these two conditions.
If the equation is simple it may be overly restrictive to impose these as it
may lead to larger inaccuracies in other regions.
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.13
At which states are the departure terms for h and s small? What is Z there?
Departure terms for h and s are small at very low pressure or at very high
temperature. In both cases, Z is close to 1 and this is the ideal gas region.
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.14
The departure functions for h and s as defined are always positive. What does that
imply for the real substance h and s values relative to ideal gas values?
Real-substance h and s are less than the corresponding ideal-gas values.
This is true for the range shown in the figures, Pr < 10. For higher P the isotherms
do bend down and negative values are possible.
Generally this means that there are slightly attractive forces between the
molecules leading to some binding energy that shows up as a negative potential
energy (thus smaller h and u) and it also give a little less chaos (smaller s) due to
the stronger binding as compared to ideal gas. When the pressure becomes large
the molecules are so close together that the attractive forces turns into repulsive
forces and the departure terms are negative.
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.15
What is the benefit of Kay’s rule versus a mixture equation of state?
Kay’s rule for a mixture is not nearly as accurate as an equation of state
for the mixture, but it is very simple to use and it is general. For common
mixtures new mixture EOS are becoming available in which case they are
preferable for a greater accuracy in the calculation.
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
Clapeyron Equation
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.16
An approximation for the saturation pressure can be ln Psat = A – B/T, where A
and B are constants. Which phase transition is that suitable for, and what kind of
property variations are assumed?
Clapeyron Equation expressed for the three phase transitions are shown in Eqs.
12.5-12.7. The last two leads to a natural log function if integrated and ideal gas
for the vapor is assumed.
dPsat
hevap
=
P
sat RT2
dT
where hevap is either hfg or hig. Separate the variables and integrate
-1
Psat dPsat = hevap R-1 T-2 dT
ln Psat = A – B/T ;
B = hevap R-1
if we also assume hevap is constant and A is an integration constant. The function
then applies to the liquid-vapor and the solid-vapor interphases with different
values of A and B. As hevap is not excactly constant over a wide interval in T it
means that the equation cannot be used for the total domain.
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.17
Verify that Clapeyron’s equation is satisfied for R-410A at 10oC in Table B.4.
Clapeyron Eq.:
B.4:
dPsat dPg hfg
dT = dT = Tvfg
P = 1085.7 kPa,
hfg = 208.57 kJ/kg,
vfg = 0.02295 m3/kg
Slope around 10oC best approximated by cord from 5oC to 15oC
dPg 1255.4 – 933.9
= 32.15 kPa/K,
dT =
15 – 5
hfg
208.57
=
Tvfg 283.15 × 0.02295 = 32.096 kPa/K
This fits very well. Use CATT3 to do from 9 to 11oC for better approximation
as the saturated pressure is very non-linear in T.
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.18
In a Carnot heat engine, the heat addition changes the working fluid from
saturated liquid to saturated vapor at T, P. The heat rejection process occurs at
lower temperature and pressure (T − ∆T), (P − ∆P). The cycle takes place in a
piston cylinder arrangement where the work is boundary work. Apply both the
first and second law with simple approximations for the integral equal to work.
Then show that the relation between ∆P and ∆T results in the Clapeyron equation
in the limit ∆T → dT.
T
T
T −∆T
1
P
P
2
P- ∆P
qH = Tsfg;
4
s
sfg at T
2
T
P- ∆P
3
4
1
P
T- ∆T
3
v
vfg at T
wnet = qH - qL = ∆Tsfg
qL = (T-∆T)sfg ;
⌠ Pdv
The boundary movement work, w = ⌡
3
4
⌠ Pdv + (P - ∆P)(v4 - v3) + ⌡
⌠ Pdv
wNET = P(v2-v1) + ⌡
2
1
Approximating,
3
∆P
⌠ Pdv ≈ (P - ) (v3 - v2);
⌡
2
2
4
∆P
⌠ Pdv ≈ (P - ) (v1 - v4)
⌡
2
1
v2+v3
v1+v4
wNET ≈ ∆P[( 2 ) - ( 2 )]
Collecting terms:
(the smaller the ∆P, the better the approximation)
sfg
∆P
≈ 1
⇒
1
∆T
(v + v ) − (v + v )
2
In the limit as
2
3
∆T → 0:
2
1
4
v3 → v2 = vg ,
v4 → v1 = vf
lim ∆P dPsat sfg
& ∆T→0 = dT = v
∆T
fg
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.19
Verify that Clapeyrons equation is satisfied for carbon dioxide at 6oC in Table
B.3.
dPsat dPg hfg
dT = dT = Tvfg
Clapeyron Eq.:
B.3:
P = 4072.0 kPa,
hfg = 211.59 kJ/kg,
vfg = 0.00732 m3/kg
Slope around 6oC best approximated by cord from 4oC to 8oC
dPg 4283.1 – 3868.8
= 103.575 kPa/K,
8–4
dT =
hfg
211.59
kJ/kg
=
Tvfg 279.15 × 0.00732 K m3/kg = 103.549 kPa/K
This fits very well.
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.20
Use the approximation given in problem 12.16 and Table B.1 to determine A and
B for steam from properties at 25oC only. Use the equation to predict the
saturation pressure at 30oC and compare to table value.
dPsat
-2
dT = Psat (-B)(-T )
so we notice from Eq.12.7 and Table values from B.1.1 and A.5 that
hfg 2442.3 kJ/kg
B = R = 0.4615 kJ/kg-K = 5292 K
ln Psat = A – B/T
⇒
Now the constant A comes from the saturation pressure as
5292
A = ln Psat + B/T = ln 3.169 + 273.15 + 25 = 18.9032
Use the equation to predict the saturation pressure at 30oC as
5292
ln Psat = A – B/T = 18.9032 - 273.15 + 30 = 1.4462
Psat = 4.2469 kPa
compare this with the table value of Psat = 4.246 kPa and we have a very accurate
approximation.
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.21
A certain refrigerant vapor enters a steady flow constant pressure condenser at
150 kPa, 70°C, at a rate of 1.5 kg/s, and it exits as saturated liquid. Calculate the
rate of heat transfer from the condenser. It may be assumed that the vapor is an
ideal gas, and also that at saturation, vf << vg. The following quantities are known
for this refrigerant:
ln Pg = 8.15 - 1000/T ;
CP = 0.7 kJ/kg K
with pressure in kPa and temperature in K. The molecular mass is 100.
Solution:
Refrigerant: State 1 T1 = 70oC P1 = 150 kPa
State 2 P2 = 150 kPa x2 = 1.0
Energy Eq.:
State 3
P3 = 150 kPa x3 = 0.0
q = h3 - h1 = (h3 - h2) + (h2 - h1) = - hfg T3 + CP0(T2 - T1)
1 3
Get the saturation temperature at the given pressure
ln (150) = 8.15 - 1000/T2 => T2 = 318.5 K = 45.3oC = T3
Now get the enthalpy of evaporation hfg T3
dPg hfg
dT = Tvfg ,
dPg
d ln Pg
hfg
=
P
=
P
g dT
dT
RT2 g
RT
vfg ≈ vg = P ,
g
d ln Pg
2
2
dT = +1000/T = hfg/RT
hfg = 1000 × R = 1000 × 8.3145/100 = 83.15 kJ/kg
Substitute into the energy equation
q = -83.15 + 0.7(45.3 - 70) = -100.44 kJ/kg
1 3
.
QCOND = 1.5(-100.44) = -150.6 kW
T
P
1
3
2
3
1
v
2
s
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.22
Calculate the values hfg and sfg for nitrogen at 70 K and at 110 K from the
Clapeyron equation, using the necessary pressure and specific volume values
from Table B.6.1.
dPg hfg sfg
Clapeyron equation Eq.12.7:
dT = Tvfg = vfg
For N2 at 70 K, using values for Pg from Table B.6 at 75 K and 65 K, and also
vfg at 70 K,
∆Pg
76.1-17.41
hfg ≈ T(vg-vf)
= 70(0.525 015)
= 215.7 kJ/kg
75-65
∆Τ
sfg = hfg/T = 3.081 kJ/kg K
(
Table B.6.1:
hfg = 207.8 kJ/kg
)
and sfg = 2.97 kJ/kg K
Comparison not very close because Pg not linear function of T. Using 71 K &
69 K from the software, we can then get
(44.56-33.24
71-69 ) = 208.0 kJ/kg
hfg = 70(0.525 015)
At 110 K,
(1938.8-1084.2
115-105 ) = 134.82 kJ/kg
hfg ≈ 110(0.014 342)
134.82
sfg = hfg/T = 110 = 1.226 kJ/kg K
Table B.6.1: hfg = 134.17 kJ/kg and sfg = 1.22 kJ/kg K
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.23
Find the saturation pressure for refrigerant R-410A at –80oC assuming it is higher
than the triple point temperature.
The lowest temperature in Table B.4 for R-410A is -60oC, so it must be extended
to -80oC using the Clapeyron Eq. 12.7 integrated as in example 12.1
Table B.4 at T1 = -60oC = 213.15 K:
P1 = 64.1 kPa, R = 0.1145 kJ/kg-K
P hfg (T - T1) 279.96 (193.15 - 213.15)
=
= -1.1878
ln P = R
T × T1 0.1145 193.15 × 213.15
1
P = 64.1 exp(-1.1878) = 19.54 kPa
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.24
Ammonia at –70oC is used in a special application at a quality of 50%. Assume
the only table available is B.2 that goes down to –50oC. To size a tank to hold 0.5
kg with x = 0.5, give your best estimate for the saturated pressure and the tank
volume.
To size the tank we need the volume and thus the specific volume. If we do not
have the table values for vf and vg we must estimate those at the lower T. We
therefore use Clapeyron equation to extrapolate from –50oC to –70oC to get the
saturation pressure and thus vg assuming ideal gas for the vapor.
The values for vf and hfg do not change significantly so we estimate
Between -50oC and –70oC:
hfg = 1430 kJ/kg
o
and at –70 C we get: vf = 0.001375 m3/kg
The integration of Eq.12.7 is the same as in Example 12.1 so we get
P2 hfg T2 - T1
1430
ln P = R ( T T ) = 0.4882
-70 + 50
= -1.2923
203.15 × 223.15
1
2 1
P2 = P1 exp(-1.2923) = 40.9 exp(-1.2923) = 11.2 kPa
0.4882 × 203.15
vg = RT2/P2 =
= 8.855 m3/kg
11.2
v2 = (1-x) vf + x vg = 0.5 × 0.001375 + 0.5 × 8.855 = 4.428 m3/kg
V2 = mv2 = 2.214 m3
P
A straight line extrapolation
will give a negative pressure.
T
-70 -50
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.25
Use the approximation given in problem 12.16 and Table B.4 to determine A and
B for refrigerant R-410A from properties at 0oC only. Use the equation to predict
the saturation pressure at 5oC and compare to table value.
dPsat
-2
dT = Psat (-B)(-T )
so we notice from Eq.12.7 and Table values from B.4.1 and A.5 that
ln Psat = A – B/T
⇒
hfg 221.37 kJ/kg
B = R = 0.1145 kJ/kg-K = 1933.4 K
Now the constant A comes from the saturation pressure as
A = ln Psat + B/T = ln 798.7 +
1933.4
273.15 = 13.7611
Use the equation to predict the saturation pressure at 5oC as
ln Psat = A – B/T = 13.7611 -
1933.4
273.15 + 5 = 6.8102
Psat = 907 kPa
compare this with the table value of Psat = 933.9 kPa and we have an
approximation 3% low. Notice hfg decreases so we could have used a lower value
for the average in the interval.
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.26
The triple point of CO2 is -56.4oC. Predict the saturation pressure at that point
using Table B.3.
The lowest temperature in Table B.3 for CO2 is -50oC, so it must be extended to
-56.4oC = 216.75 K using the Clapeyron Eq. 12.7 integrated as in Ex. 12.1
Table B.3:
at T1 = -50oC = 223.15 K,
Table A.5:
R = 0.1889 kJ/kg-K
P1 = 682.3 kPa, hfg = 339.73 kJ/kg
P hfg (T - T1) 339.73 (216.75 - 223.15)
ln P = R
=
= -0.23797
T × T1 0.1889 216.75 × 223.15
1
P = 682.3 exp(-0.23797) = 537.8 kPa
Notice from Table 3.2: P = 520.8 kPa so we are 3% high. As hfg becomes larger
for lower T’s we could have estimated a more suitable value for the interval from
-50 to -56.4oC
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.27
Helium boils at 4.22 K at atmospheric pressure, 101.3 kPa, with hfg = 83.3
kJ/kmol. By pumping a vacuum over liquid helium, the pressure can be lowered,
and it may then boil at a lower temperature. Estimate the necessary pressure to
produce a boiling temperature of 1 K and one of 0.5 K.
Solution:
Helium at 4.22 K: P1 = 0.1013 MPa,
dPSAT
hFG
dT = TvFG ≈
hFGPSAT
RT2
hFG = 83.3 kJ/kmol
P2
hFG 1
1
⇒ ln P = R [T − T ]
1
1
2
For T2 = 1.0 K:
P2
1
1
83.3
ln 101.3 = 8.3145[4.22 − 1.0]
=> P2 = 0.048 kPa = 48 Pa
For T2 = 0.5 K:
P2
1
1
83.3
ln 101.3 = 8.3145[4.22 − 0.5]
P2 = 2.1601×10-6 kPa = 2.1601 × 10-3 Pa
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.28
Using the properties of water at the triple point, develop an equation for the
saturation pressure along the fusion line as a function of temperature.
Solution:
The fusion line is shown in Fig. 2.3 as the S-L interphase. From Eq.12.5 we have
dPfusion hif
dT = Tvif
Assume hif and vif are constant over a range of T’s. We do not have any simple
models for these as function of T other than curve fitting. Then we can integrate
the above equation from the triple point (T1, P1) to get the pressure P(T) as
hif
T
P – P1 = v ln T
if
1
Now take the properties at the triple point from B.1.1 and B.1.5
P1 = 0.6113 kPa,
T1 = 273.16 K
vif = vf – vi = 0.001 – 0.0010908 = - 9.08 × 10−5 m3/kg
hif = hf – hi = 0.0 – (-333.4) = 333.4 kJ/kg
The function that approximates the pressure becomes
P = 0.6113 – 3.672 × 106 ln
T
T1
[kPa]
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.29
Using thermodynamic data for water from Tables B.1.1 and B.1.5, estimate the
freezing temperature of liquid water at a pressure of 30 MPa.
P
dPif hif
H2O dT = Tv ≈ const
if
30 MPa
T.P.
T
At the triple point,
vif = vf - vi = 0.001 000 - 0.001 090 8 = -0.000 090 8 m3/kg
hif = hf - hi = 0.01 - (-333.40) = 333.41 kJ/kg
dPif
333.41
dT = 273.16(-0.000 090 8) = -13 442 kPa/K
⇒ at P = 30 MPa,
(30 000-0.6)
T ≈ 0.01 + (-13 442) = -2.2 oC
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.30
Ice (solid water) at −3°C, 100 kPa, is compressed isothermally until it becomes
liquid. Find the required pressure.
Water, triple point T = 0.01oC , P = 0.6113 kPa
Table B.1.1: vf = 0.001 m3/kg, hf = 0.01 kJ/kg,
Tabel B.1.5:
Clapeyron
vi = 0.001 0908 m3/kg, hi = -333.4 kJ/kg
dPif
hf - hi
333.4
=
=
dT (vf - vi)T -0.0000908 × 273.16 = -13 442 kPa/K
dPif
∆P ≈ dT ∆T = -13 442(-3 - 0.01) = 40 460 kPa
P = Ptp + ∆P = 40 461 kPa
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.31
From the phase diagram for carbon dioxide in Fig. 2.5 and 2.4 for water what can
you infer for the specific volume change during melting assuming the liquid has a
higher h than the solid phase for those two substances.
The saturated pressure versus temperature has a positive slope for carbon dioxide
and a negative slope for water.
Clapeyron
dPif
hf - hi
=
dT (vf - vi)T
So if we assume hf - hi > 0 then we notice that the volume change in the melting
gives
Water:
vf - vi < 0 so vf < vi
Carbon dioxide:
vf - vi > 0
so
vf > v i
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.32
A container has a double wall where the wall cavity is filled with carbon dioxide
at room temperature and pressure. When the container is filled with a cryogenic
liquid at 100 K the carbon dioxide will freeze so that the wall cavity has a mixture
of solid and vapor carbon dioxide at the sublimation pressure. Assume that we do
not have data for CO2 at 100 K, but it is known that at −90°C: Psat = 38.1 kPa,
hIG = 574.5 kJ/kg. Estimate the pressure in the wall cavity at 100 K.
Solution:
For CO2 space: at T1 = -90 oC = 183.2 K , P1 = 38.1 kPa, hIG = 574.5 kJ/kg
For T2 = TcO2 = 100 K: Clapeyron
dPSUB hIG hIGPSUB
dT = TvIG ≈ RT2
P2 hIG
1
1
574.5
1
1
ln P = R [183.2 − 100] = 0.188 92 [183.2 − 100] = −13.81
1
or P2 = P1 × 1.005×10-6 ⇒ P2 = 3.83×10-5 kPa = 3.83×10-2 Pa
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.33
Small solid particles formed in combustion should be investigated. We would like
to know the sublimation pressure as a function of temperature. The only
information available is T, hFG for boiling at 101.3 kPa and T, hIF for melting at
101.3 kPa. Develop a procedure that will allow a determination of the sublimation
pressure, Psat(T).
TNBP = normal boiling pt T.
P
TNMP = normal melting pt T.
101.3 kPa
PTP
Solid
TTP = triple point T.
Liquid
Vap.
T
TTP TNMP T NBP
1) TTP ≈ TNMP
T
P
TP
⌠ hFG
2 dT
(1/P
)
dP
≈
⌠
SAT
SAT
⌡
⌡ RT
TP
2)
0.1013 MPa
TNMP
Since hFG ≈ const ≈ hFG NBP the integral over temperature becomes
hFG NBP 1
PTP
1
ln 0.1013 ≈ R [T
-T ]
NBP
TP
→
get PTP
3) hIG at TP = hG - hI = (hG - hF) + (hF - hI) ≈ hFG NBP + hIF NMP
Assume hIG ≈ const. again we can evaluate the integral
P
T
hIG 1
⌠ hIG
1
(1/P
)
dP
≈
dT
≈
[
− T]
ln P = ⌠
2
SUB
SUB
⌡
R
T
⌡ RT
TP
TP
PSUB
SUB
PTP
TTP
or PSUB = fn(T)
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
Property Relations
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.34
Use Gibbs relation du = Tds – Pdv and one of Maxwell’s relations to find an
expression for (∂u/∂P)T that only has properties P, v and T involved. What is the
value of that partial derivative if you have an ideal gas?
du = Tds – Pdv
divide this by dP so we get
∂u
∂s
∂v
∂v
∂v
= T – P = –T – P
∂PT
∂PT
∂PT
∂TP
∂PT
where we have used Maxwell Eq.12.19. Now for an ideal gas we get
RT
Ideal gas: Pv = RT ⇒ v = P
then the derivatives are
R
∂v
∂v
and
=P
= –RTP–2
∂T
P
∂PT
and the derivative of u is
R
∂u
∂v
∂v
= –T – P = –T P – P( –RTP–2) = 0
∂PT
∂TP
∂PT
This confirms that u is not sensitive to P and only a function of T.
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.35
The Joule-Thomson coefficient µJ is a measure of the direction and magnitude of
the temperature change with pressure in a throttling process. For any three
properties x,y,z use the mathematical relation
∂x ∂y ∂z
= –1
∂yz ∂zx ∂xy
to show the following relations for the Joule-Thomson coefficient:
∂v
T – v
∂TP
RT2 ∂Z
∂T
µJ = =
=
CP
PCP ∂TP
∂P h
Let x = T, y = P and z = h and substitute into the relations as:
∂T ∂P ∂h
= –1
∂Ph ∂h T ∂TP
∂h
Then we have the definition of specific heat as CP = so solve for the first
∂TP
term
1 ∂P
1 ∂h
∂T
µJ = = – C / = – C
∂P h
P ∂hT
P ∂PT
The last derivative is substituted with Eq.12.25 so we get
∂v
T – v
∂TP
∂T
µJ = =
CP
∂P h
If we use the compressibility factor then we get
ZR RT ∂Z
v RT ∂Z
∂v
Pv = ZRT
= P + P =T+ P
∂TP
∂TP
∂TP
so then
RT2 ∂Z
RT2 ∂Z
∂v
T –v=v + P –v= P
∂TP
∂TP
∂TP
and we have shown the last expression also.
∂v
T – v
∂TP
RT2 ∂Z
∂T
µJ = =
=
CP
PCP ∂TP
∂P h
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.36
Find the Joule-Thomson coefficient for an ideal gas from the expression given in
Problem 12.35
∂v
T – v
∂TP
RT2 ∂Z
∂T
µJ = =
=
CP
PCP ∂TP
∂P h
For an ideal gas: v = RT/P so then the partial derivative
R
RT
∂v
∂v
T –v= P –v=v–v=0
=P
∂TP
∂TP
For an ideal gas Z = 1 so the very last derivative of Z is also zero.
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.37
Start from Gibbs relation dh = Tds + vdP and use one of Maxwell’s equation to
get (∂h/∂v)T in terms of properties P, v and T. Then use Eq.12.24 to also find an
expression for (∂h/∂T)v.
Find
∂h
(∂h∂v)T and (∂T
)v
dh = Tds + vdP
⇒
and use Eq.12.18
∂P
∂P
(∂v∂h)T = T (∂v∂s )T + v(∂P
)
= T ( )v + v( )T
T
∂v
∂T
∂v
Also for the second first derivative use Eq.12.24
∂h
∂P
∂P
(∂T
)v = T(∂T∂s )v + v(∂T
)v = Cv + v(∂T
)v
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.38
From Eqs. 12.23 and 12.24 and the knowledge that Cp > Cv what can you
conclude about the slopes of constant v and constant P curves in a T-s diagram?
Notice that we are looking at functions T(s, P or v given).
Solution:
The functions and their slopes are:
∂T
Constant v: T(s) at that v with slope
∂s v
∂T
Constant P: T(s) at that P with slope
∂s P
Slopes of these functions are now evaluated using Eq.12.23 and Eq.12.24 as
∂T
∂s -1 T
= = C
∂s P ∂TP
p
-1
T
∂T ∂s
= = C
∂T
∂s
v
v
v
Since we know Cp > Cv then it follows that T/Cv > T/Cp and therefore
∂T
∂T
>
∂s v
∂s P
which means that constant v-lines are steeper than constant P lines in a T-s
diagram.
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.39
Derive expressions for (∂T/∂v)u and for (∂h/∂s)v that do not contain the properties
h, u, or s. Use Eq. 12.30 with du = 0.
∂P
P - T( )v
∂T
∂u
∂u
(∂T
)
(see Eqs. 12.33 and 12.34)
= - ( )T ( )v =
u
Cv
∂v
∂v
∂T
/
As dh = Tds + vdP => (
∂h
∂P
∂T
)
= T + v( )v = T - v( )s
v
∂s
∂s
∂v
But
∂s
∂s
(∂T
)
= - ( )T / ( )v = s
∂v
∂v
∂T
⇒
vT ∂P
(∂h
)
= T + C ( )v
v
∂s
v ∂T
∂P
)
∂T v
Cv
(Eq.12.20)
T(
(Eq.12.22)
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.40
Evaluate the isothermal changes in the internal energy, the enthalpy and the
entropy for an ideal gas. Confirm the results in Chapters 3 and 6.
We need to evaluate duT, dhT and dsT for an ideal gas: P = RT/v.
From Eq.12.31 we get
duT = [ T (
∂P
) – P ] dvT = [ T ( Rv ) – P ] dvT = [ P – P] dvT = 0
∂T v
From Eq.12.27 we get using v = RT/P
dhT = [ v – T (
∂v
R
)
] dPT = [ v – T ( P ) ] dPT = [ v – v ] dPT = 0
P
∂T
These two equations confirms the statements in chapter 5 that u and h are
functions of T only for an ideal gas.
From Eq.12.32 or Eq.12.34 we get
∂v
∂P
dsT = – ( )P dPT = ( )v dvT
∂T
∂T
R
R
= – P dPT = v dvT
so the change in s can be integrated to find
P2
v2
s2 – s1 = –R ln P = R ln v
1
1
when T2 = T1
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.41
Develop an expression for the variation in temperature with pressure in a constant
entropy process, (∂T/∂P)s, that only includes the properties P–v–T and the specific
heat, Cp. Follow the development for Eq.12.32.
∂s
(∂P
)T
(∂T
) = - ∂s
∂P s
(∂T)P
∂v
)
∂T P T ∂v
= - (C /T) = C ( )P
P
P ∂T
-(
∂s
∂v
{(∂P
)T = -(∂T
)P, Maxwell relation Eq. 12.23 and the other is Eq.12.27}
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.42
Use Eq. 12.34 to get an expression for the derivative (∂T/∂v)s. What is the general
shape of a constant s process curve in a T-v diagram? For an ideal gas can you say
a little more about the shape?
Equation 12.34 says
dT
∂P
ds = Cv T + ( )v dv
∂T
so then in a constant s process we have ds = 0 and we find
T ∂P
(∂T
)
= − C ( )v
s
∂v
v ∂T
As T is higher the slope is steeper (but negative) unless the last term (∂P/∂T)v
counteracts. If we have an ideal gas this last term can be determined
∂P
(∂T
)v = Rv
P = RT/v
⇒
T R
P
(∂T
)
=−
=
−
s
Cv v
Cv
∂v
and we see the slope is steeper for higher P and a little lower for higher T as Cv is
an increasing function of T.
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.43
Show that the P-v-T relation as P(v – b) = RT satisfies the mathematical relation
in Problem 12.35.
∂x ∂y ∂z
= –1
∂yz ∂zx ∂xy
Let (x, y, z ) be (P, v, T) so we have
∂P ∂v ∂T
= –1
∂vT ∂TP ∂Pv
The first derivative becomes, P = RT/(v – b)
∂P
= –RT (v – b)-2 = – P/(v – b)
∂vT
The second derivative, v = b + RT/P
∂v
= R/P
∂TP
The third derivative, T = (P/R)(v – b)
∂T
= (v – b)/R
∂Pv
Substitute all three derivatives into the relation
RT
R v–b
RT
1
∂P ∂v ∂T
× P × R = – (v – b) × P = – 1
=–
2
∂vT ∂TP ∂Pv
(v – b)
with the last one recognized as a rewrite of the original EOS.
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
Volume Expansivity and Compressibility
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.44
What are the volume expansivity αp, the isothermal compressibility βT, and the
adiabatic compressibility βs for an ideal gas?
The volume expansivity from Eq.12.37 and ideal gas v = RT/P gives
1 R
1 ∂v
1
αp = v( )P = v ( P ) = T
∂T
The isothermal compressibility from Eq.12.38 and ideal gas gives
1 ∂v
1
1
βT = − v( )T = − v ( − RT P−2 ) =
P
∂P
The adiabatic compressibility βs from Eq.12.40 and ideal gas
1 ∂v
βs = − v( )s
∂P
From Eq.12.32 we get for constant s (ds = 0)
v
T ∂v
T R
(∂T
)
= C ( )P = C P = C
s
∂P
p ∂T
p
p
and from Eq.12.34 we get
C ∂P
C
C
∂v
(∂T
)s = − Tv (∂T
)v = − Tv Rv = − Pv
Finally we can form the desired derivative
Cv v
∂v
∂v
v
(∂P
)s = (∂T
)s (∂T
)
=
−
P Cp = − kP
∂P s
1 ∂v
1
1
v
1
βs = − v( )s = (− v) (− ) = kP = βT
kP
k
∂P
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.45
Assume a substance has uniform properties in all directions with V = LxLyLz and
show that volume expansivity αp = 3δT. Hint: differentiate with respect to T and
divide by V.
V = LxLyLz
From Eq.12.37
αp =
∂ LxLyLz
1 ∂V
1
(
)
=
(
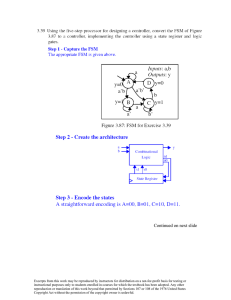
)P
V ∂T P LxLyLz
∂T
LxLz ∂ Ly
LyLz ∂ Lx
LxLy ∂ Lz
=L L L (
)
+
(
)
+
( )
x y z ∂T P LxLyLz ∂T P LxLyLz ∂T P
1 ∂ Lx
1 ∂ Ly
1 ∂ Lz
=L (
)
+
(
)
+
( )
x ∂T P Ly ∂T P Lz ∂T P
= 3 δT
This of course assumes isotropic properties (the same in all directions).
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.46
Determine the volume expansivity, αP, and the isothermal compressibility, βT, for
water at 20°C, 5 MPa and at 300°C, and 15 MPa using the steam tables.
Water at 20oC, 5 MPa (compressed liquid)
1 ∂v
1 ∆v
αP = v( )P ≈ v( )P ;
∂T
∆T
1 ∂v
1 ∆v
βT = - v( )T ≈ - v( )T
∂P
∆P
Estimate by finite difference using values at 0oC, 20oC and 40oC,
1
0.001 0056 - 0.000 9977
αP ≈ 0.000 9995
= 0.000 1976 oC-1
40 - 0
Using values at saturation, 5 MPa and 10 MPa,
1
0.000 9972 - 0.001 0022
= 0.000 50 MPa-1
βT ≈ - 0.000 9995
10 - 0.0023
Water at 300oC, 15 MPa (compressed liquid)
1
0.001 4724 - 0.001 3084
αP ≈ 0.001 377
= 0.002 977 oC-1
320 - 280
1
0.001 3596 - 0.001 3972
βT ≈ - 0.001 377
= 0.002 731 MPa-1
20 - 10
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.47
Use the CATT3 software to solve the previous problem.
The benefit of the software to solve for the partial derivatives is that we can
narrow the interval over which we determine the slope.
Water at 20oC, 5 MPa (compressed liquid)
1 ∂v
1 ∆v
αP = v( )P ≈ v( )P ;
∂T
∆T
1 ∂v
1 ∆v
βT = - v( )T ≈ - v( )T
∂P
∆P
Estimate by finite difference using values at 19oC, 20oC and 21oC,
1
0.000 9997 - 0.000 9993
αP ≈ 0.000 9995
= 0.000 40 oC-1
21 - 19
Using values at saturation, 4.5 MPa and 5.5 MPa,
1
0.000 9993 - 0.000 9997
βT ≈ - 0.000 9995
= 0.000 40 MPa-1
5.5 - 4.5
Water at 300oC, 15 MPa (compressed liquid)
1
0.001 385 - 0.001 369
= 0.011 619 oC-1
αP ≈ 0.001 377
302 - 298
1
0.001 373 - 0.001 381
βT ≈ - 0.001 377
= 0.002 905 MPa-1
16 - 14
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.48
A cylinder fitted with a piston contains liquid methanol at 20°C, 100 kPa and
volume 10 L. The piston is moved, compressing the methanol to 20 MPa at
constant temperature. Calculate the work required for this process. The isothermal
compressibility of liquid methanol at 20°C is 1.22 × 10-9 m2/N.
2
2
⌠ ∂v
⌠
w
=
Pdv
=
P
dP
=
1 2 ⌡
⌡ vβT PdPT
∂P T T ⌠
⌡
1
( )
1
For v ≈ constant & βT ≈ constant the integral can be evaluated
1w2 = -
vβT
2
(P - P )
2
2
2
1
For liquid methanol, from Table A.4: ρ = 787 m3/kg
V1 = 10 L, m = 0.01 × 787 = 7.87 kg
1W2 =
0.01×1220
(20)2 - (0.1)2
2
[
] = 2440 J = 2.44 kJ
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.49
For commercial copper at 25oC (see table A.3) the speed of sound is about 4800
m/s. What is the adiabatic compressibility βs?
From Eq.12.41 and Eq.12.40
c2 =
∂P
1
1
(∂P
)
=
−v
(
)
=
=
∂ρ s
∂v s
βρ
1 ∂v
-v( )s ρ
∂P
2
s
Then we get using density from Table A.3
1
1
s2 m 3
1000
1
=
βs = 2 =
2
2
2
kPa
m
kg
c ρ 4800 × 8300
4800 × 8300
= 5.23 × 10−9 kPa−1
Cu
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.50
Use Eq. 12.32 to solve for (∂T/∂P)s in terms of T, v, Cp and αp. How large a
temperature change does 25oC water (αp = 2.1 × 10-4 K-1) have, when
compressed from 100 kPa to 1000 kPa in an isentropic process?
From Eq.12.32 we get for constant s (ds = 0) and Eq.12.37
(∂T
) = T (∂v ) = CTp αp v
∂P s Cp ∂T P
Assuming the derivative is constant for the isentropic compression we estimate
with heat capacity from Table A.3 and v from B.1.1
∂T
T
∆Ts =
∆P
=
α v ∆Ps
∂P s s Cp p
( )
=
273.15 + 25
× 2.1 × 10-4 × 0.001003 × (1000 – 100)
4.18
= 0.013 K
barely measurable.
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.51
Sound waves propagate through a media as pressure waves that cause the media
to go through isentropic compression and expansion processes. The speed of
sound c is defined by c 2 = (∂P/∂ρ)s and it can be related to the adiabatic
compressibility, which for liquid ethanol at 20°C is 9.4 × 10-10 m2/N. Find the
speed of sound at this temperature.
c2 =
∂P
1
1
(∂P
)
= −v ( )s =
=
s
∂ρ
∂v
βρ
1 ∂v
-v( )s ρ
∂P
2
s
From Table A.4 for ethanol,
⇒
c=
ρ = 783 kg/m3
(940×101 ×783) = 1166 m/s
1/2
-12
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.52
Use Table B.3 to find the speed of sound for carbon dioxide at 2500 kPa near
100oC. Approximate the partial derivative numerically.
c2 =
(∂P
) = −v (∂P
)
∂ρ s
∂v s
2
We will use the 2000 kPa and 3000 kPa table entries. We need to find the change
in v between two states with the same s at those two pressures.
At 100oC, 2500 kPa:
s = (1.6843 + 1.5954)/2 = 1.63985 kJ/kg-K,
v = (0.03359 + 0.02182)/2 = 0.027705 m3/kg
2000 kPa, s = 1.63985 kJ/kg-K:
3000 kPa, s = 1.63985 kJ/kg-K:
c2 ≈ −v2
v = 0.031822 m3/kg
v = 0.0230556 m3/kg
3000 - 2000
kJ
J
(∆P
) = − 0.027705 0.0230556-0.031822
kg = 87 557.8 kg
∆v
2
s
c = 87 557.8 = 295.9 m/s
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.53
Use the CATT3 software to solve the previous problem.
At 100oC, 2500 kPa:
s = 1.636 kJ/kg-K, v = 0.02653 m3/kg
101oC, s = 1.636 kJ/kg-K:
99oC, s = 1.636 kJ/kg-K:
c2 ≈ −v2
v = 0.02627 m3/kg, P = 2.531 MPa
v = 0.02679 m3/kg, P = 2.469 MPa
2531 - 2469
kJ
J
(∆P
)
= − 0.02653 0.02627 - 0.02679 kg = 83 919.5 kg
∆v
2
s
c = 83 919.5 = 289.7 m/s
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.54
Consider the speed of sound as defined in Eq. 12.41. Calculate the speed of sound
for liquid water at 20°C, 2.5 MPa, and for water vapor at 200°C, 300 kPa, using
the steam tables.
From Eq. 12.41:
c2 =
∂P
(∂P
)
=
-v
(
)
∂ρ s
∂v s
2
Liquid water at 20oC, 2.5 MPa, assume
∆P
(∂P
)
≈( )
s
∂v
∆v
T
Using saturated liquid at 20oC and compressed liquid at 20oC, 5 MPa,
9995
5-0.0023
MJ
(0.001 002+0.000
) (0.000 9995-0.001
2
002) kg
2
c2 = -
J
kg
c = 1415 m/s
= 2.002×106
=>
Superheated vapor water at 200oC, 300 kPa
v = 0.7163 m3/kg, s = 7.3115 kJ/kg K
At P = 200 kPa & s = 7.3115 kJ/kg K: T = 157oC,
v = 0.9766 m3/kg
At P = 400 kPa & s = 7.3115 kJ/kg K: T = 233.8oC, v = 0.5754 m3/kg
0.400-0.200
c2 = -(0.7163)2 0.5754-0.9766
=>
c = 506 m/s
(
2 2
) MJ
kg = 0.2558 × 10 m /s
6
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.55
Use the CATT3 software to solve the previous problem.
(∂P
) = -v (∂P
)
∂ρ s
∂v s
∂P
∆P
Liquid water at 20 C, 2.5 MPa, assume ( )s ≈ ( )s
∂v
∆v
From Eq. 12.41:
c2 =
2
o
and CATT3: v = 0.001001 m3/kg, s = 0.2961 kJ/kg-K
Using liquid at 3 MPa and 2 MPa at the same s = 0.2961 kJ/kg-K,
c2 = - 0.0010012
-2
MJ
(0.001 -30.001
)
001 kg
J
kg
c = 1001 m/s
= 1.002×106
=>
Superheated vapor water at 200oC, 300 kPa
CATT3:
v = 0.7163 m3/kg, s = 7.311 kJ/kg K
At P = 290 kPa & s = 7.311 kJ/kg K: T = 196.2oC, v = 0.7351 m3/kg
At P = 310 kPa & s = 7.311 kJ/kg K: T = 203.7oC, v = 0.6986 m3/kg
0.310 - 0.290
c2 = -(0.7163)2 0.6986 - 0.7351
=>
c = 530 m/s
(
2 2
) MJ
kg = 0.28114 × 10 m /s
6
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.56
Soft rubber is used as a part of a motor mounting. Its adiabatic bulk modulus is Bs
= 2.82 × 106 kPa, and the volume expansivity is αp = 4.86 × 10-4 K-1. What is the
speed of sound vibrations through the rubber, and what is the relative volume
change for a pressure change of 1 MPa?
From Eq.12.41 and Eq.12.40
Bs
∂P
1
1
2 ∂P
=
=
c =
= −v
=
∂ρ s
∂v s
βsρ ρ
1 ∂v
-v
ρ
∂P s
2
( )
( )
( )
= 2.82 × 106 × 1000 Pa / 1100 kg/m3 = 2.564 × 106 m2/s2
c = 1601 m/s
If the volume change is fast it is isentropic and if it is slow it is isothermal. We
will assume it is isentropic
1 ∂V
1
(
)
=
−β
=
−
s
s
V ∂P
Bs
then
∆V
∆P
1000
−4
V = − Bs = − 2.82 × 106 = −3.55 × 10
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.57
Liquid methanol at 25oC has an adiabatic compressibility of 1.05 × 10-9 m2/N.
What is the speed of sound? If it is compressed from 100 kPa to 10 MPa in an
insulated piston/cylinder, what is the specific work?
From Eq.12.41 and Eq.12.40 and the density from table A.4
c2 =
∂P
1
1
(∂P
)
(
)
=
=
−v
=
∂ρ s
∂v s
β ρ 1.05 × 10-9 × 787
2
s
= 1.210 × 106 m2/s2
c = 1100 m/s
The specific work becomes
2
w = ⌠P
⌡ P dP
⌡ dv = ⌠
⌡P (-βsv ) dP = − ⌠
⌡ βsv P dP = −βs v ⌠
1
2
2
= −βs v 0.5 (P2 – P1)
0.5
= −1.05 × 10-9 m2/N × 787 m3/kg × (10 0002 – 1002) × 10002 Pa2
= −66.7 J/kg
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.58
Use Eq. 12.32 to solve for (∂T/∂P)s in terms of T, v, Cp and αp. How much higher
does the temperature become for the compression of the methanol in Problem
12.57? Use αp = 2.4 × 10-4 K-1 for methanol at 25oC.
From Eq.12.32 we get for constant s (ds = 0) and Eq.12.37
(∂T
) = T (∂v ) = CTp αp v
∂P s Cp ∂T P
Assuming the derivative is constant for the isentropic compression we estimate
with heat capacity and density (v = 1/ρ) from Table A.4
∂T
T
∆Ts =
∆P =
α v ∆Ps
∂P s s Cp p
( )
298.15 K kg K
1 m3
-4 -1
= 2.55
kJ × 2.4 × 10 K × 787 kg × (10 000 – 100) kPa
= 0.353 K
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.59
Find the speed of sound for air at 20°C, 100 kPa using the definition in Eq. 12.41
and relations for polytropic processes in ideal gases.
From problem 12.41 :
c2 =
(∂P
) = -v (∂P
)
∂ρ s
∂v s
2
For ideal gas and isentropic process, Pvk = constant
∂P
P = Cv-k ⇒
= -kCv-k-1 = -kPv-1
∂v
c2 = -v2(-kPv-1) = kPv = kRT
c = kRT = 1.4×0.287×293.15×1000 = 343.2 m/s
For every 3
seconds after the
lightning the
sound travels
about 1 km.
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
Equations of State
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.60
Use Table B3 and find the compressibility of carbon dioxide at the critical point.
Pv = Z RT
At the critical point from B.3:
P = 7377.3 kPa, T = 31°C = 304.15 K, v = 0.002139 m3/kg
from A.5:
R = 0.1889 kJ/kg-K
Pv 7377.3 × 0.002139
Z = RT = 0.1889 × 304.15 = 0.27
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.61
Use the equation of state as shown in Example 12.3 where changes in enthalpy
and entropy were found. Find the isothermal change in internal energy in a similar
fashion; do not compute it from enthalpy.
The equation of state is
Pv
P
RT = 1 – C’ T4
and to integrate for changes in u from Eq.12.31 we make it explicit in P as
v
P = T4 ( R T3 + C’ )−1
Now perform the partial derivative of P
∂P
(∂T
)v = 4 T3 ( Rv T3 + C’ )−1 − T4 ( Rv T3 + C’ )−2 3 Rv T2
P P2 v
P
P Pv P
Pv
= 4 T − 4 3 R T2 = 4 T − 3 T × RT = T [ 4 – 3 RT ]
T
Substitute into Eq.12.31
∂P
Pv
duT = [ T ( )v – P ] dvT = [ P( 4 – 3
RT) – P ] dvT
∂T
Pv
P
= 3 P ( 1 – RT) dvT = 3 P C’ 4 dvT
T
The P must be eliminated in terms of v or the opposite, we do the latter as from
the equation of state
RT
RT
1
⇒
dvT = – 2 dPT
v = P – C’ R 3
P
T
so now
P2
1
duT = 3 C’ 4 dvT = – 3 C’ R 3 dPT
T
T
and the integration becomes
u2 – u1 = − 3 C’ R T−3 (P2 – P1)
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.62
Use Table B.4 to find the compressibility of R-410A at 60oC and a) saturated
liquid b) saturated vapor and c) 3000 kPa.
Table A.2:
a)
R = 8.31451 / 72.585 = 0.1145 kJ/kg-K
Table B.4.1: P = 3836.9 kPa, v = 0.001227 m3/kg
Pv 3836.9 × 0.001227
Z = RT = 0.1145 × 333.15 = 0.1234
b)
Table B.4.1: P = 3836.9 kPa, v = 0.00497 m3/kg
Pv 3836.9 × 0.00497
Z = RT = 0.1145 × 333.15 = 0.5
c)
Table B.4.2: P = 3000 kPa, v = 0.00858 m3/kg
Pv 3000 × 0.00858
Z = RT = 0.1145 × 333.15 = 0.675
The R-410A is not an ideal gas at any of these states.
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.63
Use a truncated virial EOS that includes the term with B for carbon dioxide at 20o
C, 1 MPa for which B = −0.128 m3/kmol and T(dB/dT) = 0.266 m3/kmol. Find
the difference between the ideal-gas value and the real-gas value of the internal
energy.
R BR RT dB
RT BRT
∂P
virial eq.:
P= v + 2 ;
(
)
=
+
+ 2 (dT )
∂T v v v2
v
v
⌠v
u-u* = -
⌡∞
v
∂P
dB
RT
dB
[ (∂T
) v - P]dv = − ⌠ [ RT
(
)
]
dv = − v [T ( dT)]
dT
v
⌡
2
EA
2
A
∞
A
A
A
EA
A
A
A
E
A
E
E
Solution of virial equation (quadratic formula):
−
−
RT 8.3145×293.15
− 1 RT
−
v = 2 P 1 + 1 + 4BP/RT
where: P =
= 2.43737
1000
E
A
A
E
A
A
A
A
E
EA
E
A
[
EA
A
A
]
A
A
EA
A
E
A
A
E
E
[1 + 1 + 4(-0.128)/2.43737 ] = 2.3018 m /kmol
3
E
A
EA
E
− 1
v = 2 × 2.43737
A
A
A
A
EA
A
E
A
E
Using the minus-sign root of the quadratic formula results in a compressibility
factor < 0.5, which is not consistent with such a truncated equation of state.
u − u* =
-8.3145 × 293.15
0.266 / 44.01 = − 6.4 kJ/kg
2.3018
E
A
A
A
A
[
]
E
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.64
Solve the previous Problem with Table B.3 values and find the compressibility of
the carbon dioxide at that state.
B.3: v = 0.05236 m3/kg, u = 327.27 kJ/kg,
E
A
A
A5: R = 0.1889 kJ/kg-K
Pv 1000 × 0.05236
Z = RT = 0.1889 × 293.15 = 0.9455
A
A
A
A
E
close to ideal gas
E
To get u* let us look at the lowest pressure 400 kPa, 20oC: v = 0.13551 m3/kg
and u = 331.57 kJ/kg.
E
A
E
A
A
E
A
A
A
Z = Pv/RT = 400 × 0.13551/(0.1889 × 293.15) = 0.97883
It is not very close to ideal gas but this is the lowest P in the printed table.
u − u* = 327.27 – 331.57 = −4.3 kJ/kg
E
A
A
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.65
A gas is represented by the virial EOS with the first two terms, B and C. Find an
expression for the work in an isothermal expansion process in a piston-cylinder.
RT B(T) RT C(T) RT
P= v +
+
+ ….
v2
v3
Virial EOS:
A
A
A
A
A
A
E
E
E
E
E
= RT[ v−1 + B(T) v−2 + C(T) v−3 + .. ]
E
A
The work is
E
A
A
E
A
A
A
−1
−2
−3
w=⌠
⌡ [ v + B(T) v + C(T) v + .. ] dv
⌡ P dv = RT ⌠
A
EA
A
E
With just the first two terms we get
v2
1
−1
−1
−2
−2
w = RT [ ln v − B(T) (v2 – v1 ) − 2 C(T) (v2 – v1 ) ]
A
A
E
1
E
A
A
E
A
A
A
A
A
E
A
E
A
A
E
E
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.66
Extend problem 12.63 to find the difference between the ideal-gas value and the
real-gas value of the entropy and compare to table B.3.
Calculate the difference in entropy of the ideal-gas value and the real-gas value
for carbon dioxide at the state 20°C, 1 MPa, as determined using the virial
equation of state. Use numerical values given in Problem 12.63.
CO2 at T = 20oC, P = 1 MPa
E
A
A
A
A
E
RT/P*
RT/P*
R
P
*
sP* - sP = ⌠
v dv = R ln *
E
*
sP* - sP =
A
A
A
A
A
E
E
E
⌠ ∂P
dv ;
⌡ ∂T v
( )
ID Gas:
EA
A
A
A
A
⌡
A
E
E
EA
A
P
E
v(P)
v(P)
RT/P*
E
⌠ ∂P
P
* + ∂T v dv
P
⌡
*
Therefore, at P:
sP - sP = -R ln
A
A
A
A
E
E
A
A
( )
A
EA
E
v(P)
RT BRT
∂P
R BR RT dB
P= v + 2
and
= v + 2 + 2 dT
v
∂T
v
v
v
Integrating,
P
RT
dB
1 P*
*
sP - sP = -R ln * + R ln * + R B + T dT
v - RT
P
Pv
RT
dB 1
= R ln Pv + B + T dT v
virial:
A
A
A
( )
A
A
A
E
A
A
A
A
E
A
A
A
A
A
A
A
A
A
[
A
A
A
A
E
E
A
E
(
A
A
E
A
E
A
A
E
( )](
( )) ]
A
E
A
E
E
E
[
( )
A
E
E
E
)
A
A
A
E
E
E
A
E
Using values for CO2 from solution 12.63 and R = 0.1889 kJ/kgK
A
A
E
2.437 37
1
*
sP - sP = 0.1889 ln 2.3018 + -0.128 + 0.266 2.3018
[
A
A
A
A
E
E
A
A
(
)
]
A
E
A
E
= 0.02214 kJ/kg K
From Table B.3 take the ideal as the lowest P = 400 kPa:
*
sP - sP = 1.7904 – 1.6025 + 0.1889 ln(400/1000) = 0.0148 kJ/kgK
A
A
A
A
E
E
The lowest P = 400 kPa in B3 is not exactly ideal gas ( Z = Pv/RT = 0.9788)
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.67
Two uninsulated tanks of equal volume are connected by a valve. One tank
contains a gas at a moderate pressure P1, and the other tank is evacuated. The
A
A
E
valve is opened and remains open for a long time. Is the final pressure P2 greater
A
A
E
than, equal to, or less than P1/2? Hint: Recall Fig. 12.5.
A
A
E
Assume the temperature stays constant then for an ideal gas the pressure will be
reduced to half the original pressure. For the real gas the compressibility factor
maybe different from 1 and then changes towards one as the pressure drops.
VA = VB ⇒ V2 = 2V1, T2 = T1 = T
P2 V1 Z2 mRT
1 Z2
=
=
P V Z mRT
2Z
A
A
A
A
E
A
A
E
A
A
E
A
E
A
1
E
A
E
A
2
E
A
E
A
A
A
E
A
A
1
E
A
E
A
A
E
A
A
A
A
E
A
E
E
E
1
E
If T > TB, Z2 < Z1
A
A
E
A
Z
1T > T
B
A
E
E
1.0
1
⇒ P <2
1
A
E
A
P2
A
E
A
A
E
E
A
E
B
EVAC.
A
E
A
A
GAS
E
P2 1
If T < TB, Z2 > Z1 ⇒ P > 2
1
A
A
2
2
A
E
P2
T < T
B
1
P1 P
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.68
Show how to get the constants in Eq.12.52 for van der Waals EOS.
RT
a
− 2
v−b v
The conditions at the critical point relate to these derivatives
RT
2a
6a
∂P
∂2P
(∂v)T = − (v − b)2 + v3
;
( 2)T = (v2RT
3− 4
− b)
v
∂v
van der Waals EOS:
P=
A
A
A
E
E
A
A
E
A
A
A
A
A
A
A
A
A
E
A
A
A
A
E
E
E
E
E
E
E
Set both derivatives to zero at the critical point
−
RTc
2+
(vc − b)
A
2a
3=0
vc
2RTc
(1) ;
A
3−
(vc − b)
E
A
6a
4=0
A
vc
(2)
E
we also have from the EOS
Pc =
RTc
a
− 2
vc − b v
(3)
A
c
A
E
Now we need to solve theses three equations for vc, a and b. Solve the first
equation for a and substitute into the second equation to give
RTc
3RTc
2a
6a
=
=>
=
substitute into Eq.(2)
3
2
4
2
vc (vc − b)
vc vc (vc − b)
A
A
A
A
E
E
3RTc
2=
vc (vc − b)
2RTc
now solve to get
(vc − b)3
vc = 3b
Substitute back into the first equation to get
RTc
27
3
2a=
2 vc = RTc 4 b
(vc − b)
A
A
E
Now finally substitute a and vc into the EOS Eq.(3) to get b.
27
RTc
a RTc RTc 8 b
Pc =
− 2 = 2b −
= RTc (0.5 – 3/8) /b
vc − b v
9 b2
A
A
c
E
The result is as in Eq.12.52.
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.69
Show that the van der Waals equation can be written as a cubic equation in the
compressibility factor involving the reduced pressure and reduced temperature as
Pr
A
E
A
A
EA
E
A
r
E
A
27 Pr2
27 Pr
Z–
=0
64 T2
512 Tr 3
r
Z2 +
A
(8T + 1)
Z3 –
A
A
A
A
AE
E
A
A
EA
A
E
A
A
E
E
RT a
P = v-b - 2
v
van der Waals equation, Eq.12.55:
A
A
A
E
2
2
27 R TC
a = 64 P
C
A
A
A
E
E
A
RTC
b = 8P
A
A
EA
E
A
A
EA
AE
E
C
A
AE
v2(v-b)
multiply equation by P
A
E
Get:
E
RT
a
ab
v3 - (b + P ) v2 + (P) v - P = 0
E
A
E
A
A
A
A
A
A
A
E
Multiply by
A
A
E
E
P3
Pv
3 3 and substitute Z = RT
R T
A
A
A
E
E
Get:
E
bP
aP
abP2
2
RT + 1) Z + ( R2T2) Z – (R3 T3) = 0
Z3 – (
E
A
A
E
A
A
A
A
A
A
A
E
E
A
E
E
Substitute for a and b, get:
Pr
27 Pr2
27 Pr
Z3 – (8T + 1) Z2 +
3=0
2 Z –
512
T
r
6
4
T
r
r
A
A
A
E
A
A
E
A
EA
A
E
A
A
A
A
E
A
EA
A
AE
E
A
A
E
E
Where
P
Pr = P ,
A
A
EA
E
A
c
A
AE
T
Tr = T
A
A
EA
E
c
A
AE
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.70
Evaluate changes in an isothermal process for u, h and s for a gas with an
equation of state as P (v − b) = RT.
From Eq.12.31 we get
duT = [ T (
A
∂P
) – P ] dvT = [ T ( v R– b ) – P ] dvT = [ P – P] dvT = 0
∂T v
A
A
A
A
A
E
E
E
From Eq.12.27 we get using v = b + RT/P
dhT = [ v – T (
A
∂v
R
)
] dPT = [ v – T ( P ) ] dPT = b dPT
P
∂T
A
A
A
A
E
A
E
E
From Eq.12.32 or Eq.12.34 we get
∂v
∂P
dsT = – ( )P dPT = ( )v dvT
∂T
∂T
A
A
A
A
A
A
E
A
A
E
E
E
R
R
= – P dPT = v – b dvT
A
A
A
A
E
E
Now the changes in u, h and s can be integrated to find
u2 – u1 = 0
⌠ b dP = b(P2 – P1)
h2 – h1 = ⌡
A
EA
P2
v2 – b
s2 – s1 = –R ln P = R ln v – b
1
1
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.71
Develop expressions for isothermal changes in internal energy, enthalpy and
entropy for a gas obeying the van der Waals equation of state.
RT a
P = v-b − 2
v
van der Waals equation of state:
A
A
A
E
∂P
(∂T
)v = v-bR
∂P
RT RT a
(∂u∂v)T = T(∂T
)v - P = v-b
− v-b +
v
A
A
A
A
A
E
E
E
A
A
E
A
A
A
A
A
A
A
E
A
E
E
A
A
E
E
A
2
E
2
E
2
⌠
∂P
a
1 1
(u2-u1)T = [T
dv = a(v − v )
- P]dv = ⌠
2
v
∂T
⌡
⌡v
1
2
A
A
A
A
A
E
( )
A
A
E
E
EA
A
EA
A
A
A
A
E
1
E
1
1 1
(h2-h1)T = (u2-u1)T + P2v2 - P1v1 = P2v2 − P1v1 + a(v − v )
A
A
A
A
E
A
A
E
A
A
E
A
A
E
A
A
E
A
A
E
A
A
E
2
A
A
E
A
A
E
A
A
E
A
A
E
A
A
E
A
A
E
A
E
A
1
A
E
A
2
E
2
v -b
⌠ ∂P
⌠ R dv = R ln 2
(s2-s1)T =
dv
=
v1-b
⌡ v-b
⌡ ∂T v
A
A
E
A
A
E
A
( )
A
A
E
EA
A
EA
( )
A
E
A
1
E
1
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.72
Consider the following equation of state, expressed in terms of reduced pressure
−2
and temperature: Z = 1 + (Pr/14Tr)[1 – 6Tr ]. What does this predict for the
reduced Boyle temperature?
A
A
A
E
A
A
A
E
E
Pr
Pv
6
Z = RT = 1 + 14 T (1 - 2)
Tr
r
A
A
A
A
E
E
A
A
E
E
1
6
∂Z
= 14P T (1 - 2)
∂P
T
Tr
c r
A
A
A
A
E
E
To find the Boyle temperature we must have
Lim ∂Z
= 0 at Tboyle
P→0 ∂P T
A
A
E
The above derivative is then zero if:
(1 - T62) = 0 Tr = 6 = 2.45
r
A
A
A
A
A
EA
E
E
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.73
Use the result of Problem 12.35 to find the reduced temperature at which the
Joule-Thomson coefficient is zero, for a gas that follows the EOS given in
Problem 12.72
Pr
Pv
6
Z = RT = 1 + 14 T (1 - 2)
Tr
r
From Problem 12.35
∂v
T – v
∂TP
RT2 ∂Z
∂T
µJ = =
=
CP
PCP ∂TP
∂P h
A
A
A
A
E
E
A
A
E
E
AE
A
E
Pr -2
Pr
-2
-3
∂Z
= - 14T Tr (1 – 6 Tr ) + 14 T ( 12 Tr /Tc )
∂T P
c
r
A
A
A
A
E
E
A
E
A
E
Pr -2
-2
= 14T Tr ( 18 Tr - 1)
A
c
A
E
-2
So this is zero for 18 Tr = 1
A
or
A
E
Tr = 18
A
A
A
E
E
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.74
RT a
What is the Boyle temperature for the following equation of state: P = v-b - 2
v T
where a and b are constants.
A
A
A
E
E
RT
a
P = v-b − 2
vT
v-b
Multiplying by P gives:
Using for TBoyle:
A
A
A
E
E
A
RT a(1-b/v)
v − b = P − PvT
A
A
E
A
A
A
E
E
E
A
E
Z-1
1 lim
RT
lim ∂Z
( ) = lim
= RT P→0(v - P )
P→0 ∂P T
P→0 P-0
A
A
A
A
A
E
A
A
A
A
E
A
E
A
A
E
E
A
A
A
A
E
E
E
a(1-0)
a
(v − RT
)
=
b
−
=
b
−
= 0 at TBoyle
P
RT×T
RT2
lim
P→0
A
A
A
E
A
A
A
E
E
A
A
A
E
E
E
or
TBoyle =
A
A
E
A
a
Rb =
EA
3
2
27 R TC 1 8PC
64 PC R RTC =
E
A
EA
A
27
T
8 C
EA
A
E
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.75
Determine the reduced Boyle temperature as predicted by an equation of state (the
experimentally observed value for most substances is about 2.5), using the van der
Waals equation and the Redlich–Kwong equation. Note: It is helpful to use Eqs.
12.47 and 12.48 in addition to Eq. 12.46
lim ∂Z
The Boyle temp. is that T at which
( ) =0
P→0 ∂P T
A
A
A
A
A
A
E
E
E
1 lim
RT
lim ∂Z
lim Z-1
(
)
=
=
(
v
P→0 ∂P T
P→0 P-0
RT P→0
P)
RT a
van der Waals:
P = v-b − 2
v
v-b
multiply by P , get
RT a(v-b)
RT
a(1-b/v)
v-b = P or v - P = b − Pv
2
Pv
But
A
A
A
A
A
A
A
E
A
A
E
A
E
A
A
A
A
E
E
A
A
A
E
E
A
E
A
E
E
A
A
E
A
A
A
A
A
A
E
A
E
E
E
a(1-0)
lim ∂Z
& RT × P→0( )T = b − RT = 0
∂P
a 27
or TBoyle = Rb = 8 TC = 3.375 TC
RT
a
Redlich-Kwong:
P = v-b −
v(v+b)T1/2
as in the first part, get
RT
a(1-b/v)
v- P =b−
Pv(1+b/v)T1/2
A
A
A
A
A
A
E
A
A
E
E
E
A
E
E
A
A
A
A
E
A
E
A
E
A
A
A
E
A
E
E
only at TBoyle
A
E
A
E
E
A
A
A
E
E
E
a(1-0)
lim ∂Z
& RT × P→0( )T = b −
=0
∂P
Pv(1+0)T1/2
A
A
A
A
A
A
E
A
A
E
E
E
or
only at TBoyle
A
E
E
2 5/2
PC
a 0.427 48 R TC
3/2
TBoyle = Rb =
×
RPC
0.08 664 R TC
E
A
A
A
A
A
A
A
E
E
E
E
E
0.427 48 2/3
TBoyle = (0.086 64) TC = 2.9 TC
E
A
E
A
A
A
E
A
A
A
A
E
A
E
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.76
One early attempt to improve on the van der Waals equation of state was an
expression of the form
RT a
P = v-b - 2
vT
A
A
A
E
E
Solve for the constants a, b, and vC using the same procedure as for the van der
Waals equation.
From the equation of state take the first two derivatives of P with v:
A
A
E
RT
2a
(∂P
) = - (v-b)
2+ 3
∂v T
vT
∂2P
2RT 6a
and ( 2 )T = ∂v
(v-b)3 v4T
Since both these derivatives are zero at the critical point:
RT
2a
2RT 6a
and =0
2+ 3 =0
(v-b) v T
(v-b)3 v4T
RTC
a
Also,
PC = v -b − 2
vC TC
C
A
A
A
A
A
A
A
A
A
A
A
A
E
A
A
A
E
E
E
A
A
A
E
E
A
E
A
A
E
A
A
E
E
E
A
A
A
E
E
E
A
E
E
A
E
solving these three equations:
2 3
vC = 3b,
A
A
E
27 R TC
a = 64 P ,
C
E
A
A
E
A
E
A
RTC
b = 8P
A
E
C
E
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.77
Develop expressions for isothermal changes in internal energy, enthalpy and
entropy for a gas obeying Redlich-Kwong equation of state.
Redlich-Kwong equation of state:
P=
RT
a
−
v − b v(v + b)T1/2
A
A
A
E
E
∂P
(∂T
)v = v R− b + 2v(v +ab)T
A
A
A
A
A
A
3/2
A
E
E
E
E
From Eq.12.31
2
v2 + b
v1
3a
−3a
(u2 − u1)T = ⌠
dv
=
ln
[(
)
(
2v(v + b)T1/2
1/2
v2
v1 + b)]
2bT
⌡
A
A
A
A
A
E
A
A
E
AE
A
A
A
A
E
E
E
A
A
E
E
E
1
We find change in h from change in u, so we do not do the derivative in
Eq.12.27. This is due to the form of the EOS.
v2 + b
v1
3a
(h2 − h1)T = P2v2 − P1v1 −
1/2 ln
v2
v1 + b
2bT
A
A
A
A
A
E
A
A
E
E
A
A
A
A
E
E
A
A
E
[(
A
A
A
E
)(
A
A
E
)]
A
E
A
E
E
E
Entropy follows from Eq.12.35
2
⌠ R
a/2
(s2 − s1)T =
+
3/2 dv
⌡ v − b v(v + b)T
A
A
A
A
E
A
A
E
[
A
E
]
E
E
1
(vv −− bb) − 2bTa ln[(v v+ b)(v v+ b)]
2
= R ln
A
2
A
E
1
A
3/2
A
E
1
A
A
E
E
2
E
A
E
A
1
E
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.78
Determine the second virial coefficient B(T) using the van der Waals equation of
state. Also find its value at the critical temperature where the experimentally
observed value is about –0.34 RTc/Pc.
A
A
A
E
A
E
RT
where Eq. 12.44: α = P − v
lim
B(T) = - P→0 α
From Eq.12.48:
A
A
A
E
E
From Eq. 12.51:
RT a
v-b
P = v-b - 2
which we can multiply by P , get
v
RT a(v-b)
RT
a(1-b/v)
v-b= P −
or v − P = b − Pv
2
Pv
van der Waals:
A
A
A
A
A
E
A
E
E
A
A
A
A
A
A
E
E
E
A
E
E
E
Taking the limit for P -> 0 then (Pv -> RT and v -> ∞ ) we get :
RTC 1 27 TC
B(T) = b − a/RT = P ( 8 − 64 T )
C
A
A
E
where a,b are from Eq.12.52. At T = TC then we have
RTC 19
RTC
B(TC) = P ( - 64) = −0.297 P
A
A
A
E
C
E
A
C
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.79
Determine the second virial coefficient B(T) using the Redlich-Kwong equation
of state. Also find its value at the critical temperature where the experimentally
observed value is about –0.34 RTc/Pc.
A
A
A
E
A
E
RT
where Eq.12.44: α = P − v
lim
B(T) = - P→0 α
From Eq.12.48:
A
A
A
E
E
For Redlich Kwong the result becomes
RT
a(1- b/v)
v− P =b−
Pv(1 + b/v) T1/2
A
A
A
A
E
E
Taking the limit for P -> 0 then (Pv -> RT and v -> ∞ ) we get :
a
=> B(T) = b −
RT3/2
Now substitute Eqs. 12.54 and 12.55 for a and b,
RTC
TC3/2
B(T) = P
0.08664 - 0.42748 T
C
A
E
[
A
]
AE E
and evaluated at TC it becomes
RTC
RTC
B(TC) = P
0.08664 - 0.42748 = −0.341 P
C
[
]
C
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.80
Oxygen in a rigid tank with 1 kg is at 160 K, 4 MPa. Find the volume of the tank
by iterations using the Redlich-Kwong EOS. Compare the result with the ideal
gas law.
For the ideal gas law:
Pv = RT
so v = RT/P
v = 0.2598 × 160 / 4000 = 0.0104 m3/kg ; V = mv = 0.0104 m3
E
A
A
E
A
For Redlich-Kwong, Eq.12.53 and oxygen
Pc = 5040 kPa;
Tc = 154.6 K;
R = 0.2598 kJ/kg K
RTc
0.2598 × 154.6
b = 0.08664 P = 0.08664 ×
= 0.000 690 5 m3/kg
5040
c
A
A
A
A
E
E
E
A
A
E
A
A
E
A
A
E
5/2
R2Tc
0.25982 × 154.65/2
a = 0.427 48 P = 0.427 48 ×
= 1.7013
5040
c
E
A
A
A
E
P=
RT
a
−
v − b v(v + b)T1/2
A
A
A
E
E
A
trial and error to get v due to nonlinearity
A
E
E
v = 0.01 m3/kg ⇒
P = 4465.1 – 1279.9 = 3185.2 kPa too low
3
v = 0.008 m /kg ⇒ P = 5686.85 – 1968.1 = 3718.8 kPa too low
v = 0.0075 m3/kg ⇒ P = 6104.41 – 2227.43 = 3876.98 kPa
v = 0.007 m3/kg ⇒ P = 6588.16 – 2541.70 = 4046.46 kPa
E
A
A
E
A
A
E
A
A
E
A
A
Now we interpolate between the last two entries and check
v = 0.00714 m3/kg ⇒ P = 6445.15 – 2447.3 = 3997.8 kPa OK
V = mv = 0.00714 m3 (69% of the ideal gas value)
E
A
A
E
A
A
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.81
A flow of oxygen at 230 K, 5 MPa is throttled to 100 kPa in a steady flow
process. Find the exit temperature and the specific entropy generation using
Redlich-Kwong equation of state and ideal gas heat capacity. Notice this becomes
iterative due to the non-linearity coupling h, P, v and T.
C.V. Throttle. Steady single flow, no heat transfer and no work.
Energy eq.:
h1 + 0 = h2 + 0
A
A
A
so constant h
A
E
E
Entropy Eq.: s1 + sgen = s2
A
A
A
A
E
A
E
so entropy generation
A
E
Find the change in h from Eq.12.26 assuming Cp is constant.
A
A
E
Redlich-Kwong equation of state:
RT
a
−
v − b v(v + b)T1/2
P=
A
A
A
E
E
∂P
(∂T
)v = v R− b + 2v(v +ab)T
A
A
A
A
A
A
A
E
E
3/2
E
E
From Eq.12.31
2
v2 + b
v1
3a
−3a
(u2 − u1)T = ⌠
dv
=
ln
[(
)
(
1/2
v2
v1 + b)]
2bT1/2
⌡ 2v(v + b)T
A
A
A
A
A
E
A
A
E
AE
A
A
A
A
A
A
E
E
E
E
E
E
1
We find change in h from change in u, so we do not do the derivative in
Eq.12.27. This is due to the form of the EOS.
v2 + b
v1
3a
(h2 − h1)T = P2v2 − P1v1 −
ln
v2
v1 + b
2bT1/2
A
A
A
A
E
A
A
E
A
A
E
A
E
A
A
A
E
A
E
A
A
[(
A
E
)(
A
A
E
)]
A
E
A
E
E
E
Entropy follows from Eq.12.35
2
⌠ R
a/2
(s2 − s1)T =
+
3/2 dv
⌡ v − b v(v + b)T
A
A
E
A
A
A
A
[
A
E
E
]
E
E
1
(vv −− bb) − 2bTa ln[(v v+ b)(v v+ b)]
2
= R ln
2
A
A
1
E
A
3/2
A
1
A
A
E
E
E
2
E
A
E
A
1
E
Tc = 154.6 K;
R = 0.2598 kJ/kg K
Pc = 5040 kPa;
RTc
0.2598 × 154.6
b = 0.08664 P = 0.08664 ×
= 0.000 690 5 m3/kg
5040
c
A
A
A
E
A
E
E
A
A
E
A
A
E
A
A
E
5/2
R2Tc
0.25982 × 154.65/2
a = 0.427 48 P = 0.427 48 ×
= 1.7013
5040
c
E
A
A
E
A
A
E
E
We need to find T2 so the energy equation is satisfied
A
A
E
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
h2 – h1 = h2 – hx + hx – h1 = Cp(T2 – T1) + (h2 − h1)T = 0
A
A
A
A
A
E
A
E
A
A
E
A
A
E
A
A
E
A
A
E
A
A
E
A
E
A
A
E
A
A
E
A
A
A
E
E
and we will evaluate it similar to Fig. 12.4, where the first term is done from state
x to 2 and the second term is done from state 1 to state x (at T1 = 230 K). We do
this as we assume state 2 is close to ideal gas, but we do not know T2.
We first need to find v1 from the EOS, so guess v and find P
A
A
E
A
A
E
A
A
E
3
⇒ P = 5796.0 – 872.35 = 4924
v1 = 0.011 m /kg
A
A
E
A
A
E
too low
v1 = 0.01082 m /kg ⇒ P = 5899.0 – 900.7 = 4998.3 OK
Now evaluate the change in h along the 230 K from state 1 to state x, that requires
a value for vx. Guess ideal gas at Tx = 230 K,
3
A
A
A
E
A
E
A
A
A
E
A
E
vx = RTx/P2 = 0.2598 × 230/100 = 0.59754 m3/kg
From the EOS:
P2 = 100.1157 – 0.3138 = 99.802 kPa (close)
A few more guesses and adjustments gives
vx = 0.59635 m3/kg; P2 = 100.3157 – 0.3151 = 100.0006 kPa OK
E
A
A
A
A
E
A
A
E
A
A
E
A
A
E
E
A
A
A
A
A
A
E
E
3a
ln
2bT1/2
(hx − h1)T = Pxvx − P1v1 −
A
A
A
A
E
A
E
A
A
A
E
A
A
E
A
A
E
A
E
A
A
E
A
E
[(v v+ b)(v v+ b)]
x
1
A
A
x
A
E
E
A
1
E
E
0.59704 0.01082
= 59.635 – 5000 × 0.01082 – 243.694 ln [
×
]
0.59635 0.01151
= 59.635 – 54.1 + 14.78335 = 20.318 kJ/kg
A
A
A
A
E
E
From energy eq.: T2 = T1 – (hx − h1)T/Cp = 230 – 20.318 / 0.922 = 208 K
Now the change in s is done in a similar fashion,
A
A
A
E
A
A
A
E
A
E
A
A
A
E
A
E
A
E
sgen = s2 – s1 = (sx − s1)T + s2 – sx
A
A
E
A
A
A
A
E
A
E
A
A
E
A
A
E
A
A
E
A
A
E
E
(vv −− bb) − 2bTa ln[(v v+ b)(v v+ b)] + C ln TT
x
= R ln
x
A
E
A
1
A
3/2
A
1
A
A
E
E
E
x
E
2
A
E
A
1
E
p
A
A
A
E
A
x
E
E
0.59566
208
= 0.2598 ln(0.0101295) – 0.35318 ln (0.94114) + 0.922 ln(230)
A
A
A
E
A
E
= 1.05848 + 0.021425 – 0.092699
= 0.987 kJ/kg K
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
Generalized Charts
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.82
How low should the pressure be so that nitrous oxide (N2O) gas at 278.6 K can be
treated as an ideal gas with 5% accuracy or better?
A
A
E
From Table A.2:
Tc = 309.6 K,
A
A
E
278.6
Pc = 7.24 MPa => Tr1 = 309.6 = 0.9
A
A
A
A
A
A
E
E
E
Look in Fig. D.1 following the curve Tr1 = 0.9 to the point where Z = 0.95
A
A
E
Pr1 = 0.125
A
A
E
so
P < 0.125 × 7.24 MPa = 0.9 MPA = 900 kPa
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.83
Nitrous oxide (N2O) at 278.6 K is at a pressure so that it can be in a two-phase
state. Find the generalized enthalpy departure for the two saturated states of liquid
and vapor.
A
A
E
From Table A.2:
278.6
Pc = 7.24 MPa => Tr1 = 309.6 = 0.9
Tc = 309.6 K,
A
A
A
E
A
A
A
A
A
E
E
E
From Fig. D.2:
Saturated liquid:
Saturated vapor:
(h*- hf)/RTc = 4.08;
E
A
A
A
A
A
A
E
E
*
(h - hg)/RTc = 0.875
A
E
A
A
A
E
A
A
E
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.84
Find the heat of evaporation, hfg, for R-134a at 0°C from the generalized charts
and compare to the value in Table B.5.
A
A
E
From Table A.2:
273.15
Pc = 4.06 MPa => Tr1 = 374.2 = 0.73
Tc = 374.2 K,
A
A
A
E
A
A
A
A
A
E
E
E
From Fig. D.2:
(h*- hf)/RTc = 4.7;
Saturated liquid:
E
A
A
A
A
A
A
E
E
*
(h - hg)/RTc = 0.3
Saturated vapor:
E
A
A
A
A
E
A
A
E
hfg = hg - hf = RTc [-0.3 – (-4.7)] = 4.4 RTc
A
A
E
A
A
E
A
A
A
A
E
A
E
A
E
= 4.4 × 0.08149 × 374.2 = 134.2 kJ/kg
Table B.5.1: hfg = 198.36 kJ/kg
A
A
E
The approximation is not very good and can be improved by using the
accentric factor.
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.85
A 200-L rigid tank contains propane at 9 MPa, 280°C. The propane is then
allowed to cool to 50°C as heat is transferred with the surroundings. Determine
the quality at the final state and the mass of liquid in the tank, using the
generalized compressibility chart, Fig. D.1.
Propane C3H8: V = 0.2 m3, P1 = 9 MPa, T1 = 280oC = 553.2 K
E
A
A
A
A
A
E
E
A
A
E
A
A
A
A
E
A
E
cool to T2 = 50 oC = 323.2 K
E
A
A
A
A
E
From Table A.2:
Tc = 369.8 K,
A
Pc = 4.25 MPa
A
A
E
A
E
9
553.2
Pr1 = 4.25 = 2.118, Tr1 = 369.8 = 1.496
A
A
A
A
E
v2 = v1 =
A
A
A
A
E
A
E
A
A
E
E
Z1RT1
P1
A
E
A
From Fig. D.1: Z1 = 0.825
A
A
A
E
E
0.825×0.188 55×553.2
= 0.00956 m3/kg
9 000
=
E
A
E
A
A
A
E
From Fig. D.1 at Tr2 = 0.874,
A
A
E
PG2 = 0.45 × 4250 = 1912 kPa
A
A
E
vG2 = 0.71 × 0.188 55 × 323.2/1912 = 0.02263 m3/kg
E
A
A
A
A
E
vF2 = 0.075 ×0.188 55× 323.2/1912 = 0.00239 m3/kg
E
A
A
A
A
E
0.00956 = 0.002 39 + x2(0.02263 - 0.00239) =>
A
A
E
x2 = 0.354
A
A
E
mLIQ 2 = (1-0.354)×0.2/0.00956 = 13.51 kg
A
A
E
These tanks
contain liquid
propane.
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.86
A rigid tank contains 5 kg of ethylene at 3 MPa, 30°C. It is cooled until the
ethylene reaches the saturated vapor curve. What is the final temperature?
V = const m = 5 kg
P1 = 3 MPa T1 = 30 oC = 303.2 K
C2 H4
E
A
A
A
E
T
A
E
cool to x2 = 1.0
3
Pr1 = 5.04 = 0.595,
A
1
A
E
A
A
A
A
E
2
Final state: x = 1, v2 = v1
A
E
Fig. D.1:
v
A
A
A
303.2
Tr1 = 282.4 = 1.074
A
A
A
A
E
E
Z1 = 0.82
A
A
E
A
E
E
Pv = ZRT so take the ratio between the two states
Z2Tr2
ZG2Tr2
Pr2 = Pr1 Z T = 0.595
= 0.6756 ZG2Tr2
0.82×1.074
1 r1
A
A
A
A
E
E
A
A
E
E
A
A
A
A
E
E
A
E
E
Trial & error, Table D.4 may be easier to use than Fig. D.1:
Tr2
ZG2
0.866
0.72
A
A
E
A
A
E
Pr2
Pr2 CALC
0.42
0.421
A
A
E
A
E
~ OK
=> T2 = 244.6 K
A
A
E
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.87
The new refrigerant R-152a is used in a refrigerator with an evaporator
temperature of –20oC and a condensing temperature of 30oC. What are the high
and low pressures in this cycle?
E
A
E
A
A
A
Since we do not have the printed tables for R-152a we will use generalized charts.
The critical properties are: Tc = 386.4 K, Pc = 4.52 MPa.
A
A
A
E
A
E
Evaporator:
Tr1 = T/Tc = (273.15 – 20)/386.4 = 0.655
A
A
A
A
E
E
PG T1 = Pr1 sat Pc = 0.06 × 4.52 = 0.271 MPa
Fig. D.1:
A
A
A
A
A
A
E
E
E
Condenser:
Tr2 = T/Tc = (273.15 + 30)/386.4 = 0.785
A
A
A
A
E
E
Fig. D.1:
PG T2 = Pr2 sat Pc = 0.22 × 4.52 = 0.994 MPa
A
A
A
A
A
A
E
E
E
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.88
A 4-m3 storage tank contains ethane gas at 10 MPa, 100oC. Use Lee-Kesler EOS
and find the mass of the ethane.
E
E
A
A
A
A
The Lee-Kesler EOS is shown as the generalized charts.
Table A.2: Tc = 305.4 K, Pc = 4.88 MPa,
The reduced properties are:
A
A
A
A
E
E
10
Pr1 = 4.88 = 2.05,
A
A
E
A
373.15
Tr1 = 305.4 = 1.22
A
A
A
E
A
A
A
E
E
PV
m = ZRT =
Table A.5: R = 0.2765 kJ/kg-K
A
Fig. D.1: Z = 0.56
A
E
10 000 × 4
= 692.3 kg
0.56 × 0.2765 × 373.15
A
E
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.89
The ethane gas in the storage tank from the previous problem is cooled to 0oC.
Find the new pressure.
E
A
273.15
Tr2 = 305.4 = 0.8944
The new final state is given by: (T2, v2 = v1)
A
A
E
A
A
A
E
A
A
A
E
A
A
A
E
E
Since Z and P are unknown this becomes trial and error solution.
P2 / Z2 = mRT2 /V = 692.3 × 0.2765 × 273.15 /4 = 13071.7 kPa
A
A
A
A
E
A
E
A
E
Assume it is saturated
Pr2 = 0.53 (see Fig. D.1), Zg = 0.67, Zf = 0.09
A
A
A
A
E
A
A
E
E
P2 = 0.53 × 4880 = 2586 kPa and
A
A
E
Z2 = P2 / 13071.7 = 0.198 (two phase OK)
A
A
A
A
E
E
Ans.: P2 = 2586 kPa
A
A
E
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.90
Use CATT3 to solve the previous two problems when the acentric factor is used
to improve the accuracy.
Problem 12.88:
The Lee-Kesler EOS is shown as the generalized charts.
Table A.2: Tc = 305.4 K, Pc = 4.88 MPa, Table A.5: R = 0.2765 kJ/kg-K
Table D.4: ω = 0.099
The reduced properties are:
A
A
A
A
E
E
10
Pr1 = 4.88 = 2.05,
A
A
A
373.15
Tr1 = 305.4 = 1.22
A
E
A
PV
m = ZRT =
A
A
E
A
A
A
E
E
A
CATT3: Z = 0.605
E
10 000 × 4
= 640.8 kg
0.605 × 0.2765 × 373.15
A
E
Problem 12.89:
273.15
Tr2 = 305.4 = 0.8944
The new final state is given by: (T2, v2 = v1)
A
A
A
A
E
A
A
E
A
A
E
A
A
E
E
Since Z and P are unknown this becomes trial and error solution.
P2 / Z2 = mRT2 /V = 640.8 × 0.2765 × 273.15 /4 = 12099.3 kPa
A
A
A
A
E
A
E
A
E
Assume it is saturated vapor
Pr2 = 0.483 (CATT3), Zg = 0.69, Zf = 0.078
A
A
A
A
E
A
A
E
E
P2 = 0.483 × 4880 = 2357 kPa and
A
A
E
Z2 = P2 / 12 099.3 = 0.1948 (two phase OK)
A
A
A
A
E
E
Ans.: P2 = 2357 kPa
A
A
E
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.91
A geothermal power plant uses butane as saturated vapor 80oC into the turbine
and the condenser operates at 30oC. Find the reversible specific turbine work.
E
A
A
E
A
A
C4H10 cycle
1
A
A
A
A
E
.
WT
Turbine
E
T1 = 80 oC, x1 = 1.0 ; T3 = 30oC, x3 = 0.0
353.2
Tr1 = 425.2 = 0.831
From D.1, D.2 and D.3:
P1 = 0.325 × 3800 = 1235 kPa
E
A
A
A
A
A
2
A
A
A
E
A
A
A
E
A
E
Ht.
Exch
A
A
E
A
.
QH
E
A
E
E
A
A
E
*
(h1-h1) = 0.143 04×425.2×0.56 = 34.1
*
(s1-s1) = 0.143 04×0.475 = 0.0680
Cond
A
A
A
A
E
E
P
A
A
A
A
E
4
.
-WP
3
E
303.2
Tr3 = 425.2 = 0.713
A
A
A
A
E
E
From D.1, D.2 and D.3: P3 = 0.113×3800 = 429 kPa
A
A
E
sat. liq.: (h*- hf) = RTc×4.81 = 292.5 ;
(s*- sf) = R×6.64 = 0.950
sat. vap.: (h*- hg) = RTc×0.235 = 14.3 ;
(s*- sg) = R×0.22 = 0.031
E
A
A
A
A
A
E
E
A
A
E
A
A
A
A
E
A
A
E
E
A
A
E
A
A
E
A
A
A
E
Because of the combination of properties of C4H10 (particularly the large CP0/R),
A
A
A
A
E
A
A
E
E
s1 is larger than sg at T3. To demonstrate,
A
A
A
A
A
A
E
E
E
353.2
1235
* *
(s1-sg3) = 1.7164 ln 303.2 - 0.143 04 ln 429 = 0.1107
A
A
A
A
A
E
A
A
A
E
E
E
(s1-sg3) = -0.0680 + 0.1107 + 0.031 = +0.0737 kJ/kg K
A
A
A
A
E
E
T
1
so that T2S will be > T3, as shown in the T-s
diagram. A number of other heavy hydrocarbons
also exhibit this behavior.
A
A
E
A
3
2s 2
A
E
s
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
Solution 12.91 Continued.
Assume T2S = 315 K,
=> Tr2S = 315 / 425.2 = 0.741
From D.2 and D.3:
*
(h2S - h2S) = RTc×0.21 = 12.8
and
*
(s2S - s2S) = R×0.19 = 0.027
353.2
1235
*
*
(s1 - s2S) = 1.7164 ln 315 - 0.143 04 ln 429 = +0.0453
(s1 - s2S) = -0.0680 + 0.0453 + 0.027 ≈ 0
⇒ T2S = 315 K
*
*
(h1 - h2S) = 1.7164(353.2-315) = 65.6
wST = h1 - h2S = -34.1 + 65.6 + 12.8 = 44.3 kJ/kg
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.92
Consider the following EOS, expressed in terms of reduced pressure and
temperature:
Pr
6
Z = 1 + 14 T (1 - 2 )
Tr
r
What does this equation predict for enthalpy departure from the ideal gas value at
the state Pr = 0.4, Tr = 0.9 ? What is it from the generalized charts?
Pr
Pv
6
Z = RT = 1 + 14 T (1 - 2)
Tr
r
3
6Tc2
RT RTc
v = P + 14P (1 - 2 ) ;
T
c
R 12RTc
∂v
=P+
∂Tp
14PcT3
3
RTc 18RTc
∂v
v - T = 14P 2
∂Tp
c 14PcT
Now Eq.12.27 is integrated with limits similar to Eq.12.62
P
RTc
18
∂v
h − h* = ⌠ [v − T ] dP = 14 (1 − 2) Pr = −0.606 RTc
⌡
∂Tp
Tr
0
Evaluate at Pr = 0.4, Tr = 0.9 from Fig. D2 to get
h − h* = − (h*− h) ≈ −0.6 RTc
This result matched with in the accuracy the figure can be read.
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.93
Consider the following equation of state, expressed in terms of reduced pressure
and temperature:
Pr
6
Z = 1 + 14 T (1 - 2 )
Tr
r
What does this equation predict for entropy departure from the ideal gas value at
the state Pr = 0.4, Tr = 0.9 ?
The entropy departure is the change in s for a real gas minus the change in s
for an ideal gas, so from Eq.12.32 and eq.6.14 we get
d(s - s*) = Cp
dT ∂v
dT R
R ∂v
dP
[
C
dP
]
=
[
p
T ∂T p
T P
P − ∂T p] dP
Solve now for v from the compressibility factor ( Z = Pv/RT) to get
Pr
Pv
6
Z = RT = 1 + 14 T (1 − 2)
Tr
r
6Tc2
RT RTc
v = P + 14P (1 − 2 ) ;
T
c
3
R 12RTc
∂v
=P+
∂T p
14PcT3
3
P
12RTc
R ∂v
6 Pr
s - s* = ⌠ [ P - ] dP = ⌠ [ −
]
dP
=
−
7 R T3
⌡
⌡
∂T p
14PcT3
P
0
0
r
Evaluate at Pr = 0.4, Tr = 0.9 to get
s - s* = −0.4703 R
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.94
A very low temperature refrigerator uses neon. From the compressor the neon at
1.5 MPa, 80 K goes through the condenser and comes out at saturated liquid 40 K.
Find the specific heat transfer using generalized charts.
80
1.5
State 1: 80 K, 1.5 MPa : Tr1 = 44.4 = 1.802, Pr1 = 2.76 = 0.543
State 2: 40 K, x = 0:
Tr2 = 0.90, Pr2 = 0.532
The enthalpy departure chart Fig. D.2:
(h*- h)1 = 0.22 RTc ,
*
(h*- h)2 = 4.10 RTc
*
h2 - h1 = 1.03 (40 - 80) = -41.2 kJ/kg
*
*
h2 - h1 = h2 - h1 - (h*- h)2 + (h*- h)1
= -41.2 + 0.412 × 44.4 (-4.10 + 0.22) = -112.2 kJ/kg
q = h2 - h1 = -112.2 kJ/kg
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.95
Repeat the previous problem using CATT3 software for the neon properties.
From CATT3:
h1 = 138.3 kJ/kg,
h2 = 30.24 kJ/kg (P = 1.46 MPa)
q = h2 - h1 = 30.24 – 138.3 = -108.1 kJ/kg
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.96
A piston/cylinder contains 5 kg of butane gas at 500 K, 5 MPa. The butane
expands in a reversible polytropic process to 3 MPa, 460 K. Determine the
polytropic exponent n and the work done during the process.
C4H10 m = 5 kg T1 = 500 K P1 = 5 MPa
Rev. polytropic process:
n
n
P1V1 = P2V2
500
5
Tr1 = 425.2 = 1.176, Pr1 = 3.8 = 1.316
From Fig. D.1:
3
460
=
1.082,
P
=
= 0.789 From Fig. D.1:
r2 3.8
425.2
mZRT 5 × 0.68 × 0.1430 × 500
V1 = P =
= 0.0486 m3
5000
Tr2 =
Z1 = 0.68
Z2 = 0.74
mZRT 5 × 0.74 × 0.1430 × 460
= 0.0811 m3
3000
P =
Solve for the polytropic exponent, n, as
5
0.0811
n = ln(P1/P2) / ln(V2/V1) = ln ( ) / ln (0.0486) = 0.9976
3
V2 =
2
W = ⌠ PdV =
1 2 ⌡
1
P2V2 - P1V1 3000×0.0811 - 5000×0.0486
= 125 kJ
=
1-n
1 - 0.9976
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.97
Calculate the heat transfer during the process described in Problem 12.96.
From solution 12.96,
V1 = 0.0486 m3, V2 = 0.0811 m3,
1
W2 = 125 kJ
500
5
Tr1 = 425.2 = 1.176, Pr1 = 3.8 = 1.316
From Fig. D.1:
Z1 = 0.68
Tr2 = 1.082, Pr2 = 0.789, T2 = 460 K
From Fig. D.2:
*
(h*- h)1 = 1.30 RTC ,
(h*- h)2 = 0.90 RTC
*
h2 - h1 = 1.716(460 - 500) = -83.1 kJ/kg
8.3145×425.2
(-0.90 + 1.30) = -58.8 kJ/kg
58.124
U2 - U1 = m(h2 - h1) - P2V2 + P1V1
h2 - h1 = -83.1 +
= 5(-58.8) – 3000 × 0.0811 + 5000 × 0.0486 = -288.3 kJ
Q = U2 - U1 + 1W2 = -174.3 kJ
1 2
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.98
An ordinary lighter is nearly full of liquid propane with a small amount of vapor,
the volume is 5 cm3, and temperature is 23°C. The propane is now discharged
slowly such that heat transfer keeps the propane and valve flow at 23°C. Find the
initial pressure and mass of propane and the total heat transfer to empty the
lighter.
Propane C3H8
T1 = 23oC = 296.2 K = constant,
V1 = 5 cm3 = 5×10-6 m3,
x1 = 0.0
Tr1 = 296.2/369.8 = 0.804
From Figs. D.1 and D.2,
P1 = PG T1 = 0.25×4.25 = 1.063 MPa,
Z1 = 0.04
*
(h1-h1) = 0.188 55×369.8×4.51 = 314.5
P1V1
m1 = Z RT =
1
1
1063×5×10-6
= 0.00238 kg
0.04×0.188 55×296.2
State 2: Assume vapor at 100 kPa, 23oC ideal gas so no corrections
Therefore, m2 much smaller than m1 ( ∼ 9.0 × 10-6 kg)
QCV = m2u2 − m1u1 + mehe
= m2h2 − m1h1 − (P2−P1)V + (m1−m2)he
= m2(h2−he) + m1(he−h1) − (P2−P1)V
(he − h1) = 0 + 0 + 314.5
QCV = ≈ 0 + 0.00238(314.5) − (100 −1063)×5×10-6 = 0.753 kJ
Actual lighters use
butane and some
propane.
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.99
250-L tank contains propane at 30°C, 90% quality. The tank is heated to 300°C.
Calculate the heat transfer during the process.
V = 250 L = 0.25 m3
T1 = 30 oC = 303.2 K, x1 = 0.90
C3 H8
T
2
Heat to T2 = 300 oC = 573.2 K
M = 44.094, Tc = 369.8 K, Pc = 4.25 MPa
R = 0.188 55, CP0 = 1.6794
1
v
Tr1 = 0.82 → Fig. D.1:
Z1 = (1- x1) Zf1 + x1 Zg1 = 0.1 × 0.05 + 0.9 × 0.785 = 0.711
*
h1-h1
A
EA
A
A
Fig D.2:
SAT
A
A
AE
SAT
= 0.30 P1
A
A
E
m=
A
RTc = 0.1 × 4.43 + 0.9 × 0.52 = 0.911
E
Pr
A
A
E
= 1.275 MPa
1275×0.25
= 7.842 kg
0.711×0.188 55×303.2
A
A
E
7.842×Z2×0.188 55×573.2
Pr2 =
Z2
= 1.254
0.25×4250
at Tr2 = 1.55 Trial and error on Pr2
A
A
A
A
E
E
A
E
A
A
E
E
A
E
E
Pr2 = 0.743 => P2 = 3.158 MPa, Z2 = 0.94 , (h*- h)2 = 0.35 RTC
E
A
A
A
A
E
*
A
E
*
A
A
A
E
A
E
(h2-h1) = 1.6794(300-30)
A
A
E
A
A
A
E
A
E
= 453.4 kJ/kg
*
(h1-h1) = 0.911×0.188 55×369.8 = 63.5 kJ/kg
A
A
A
A
E
E
*
(h2-h2) = 0.35×0.188 55×369.8 = 24.4 kJ/kg
A
A
A
A
E
E
Q12 = m(h2-h1) - (P2-P1)V = 7.842(-24.4+453.4+63.5) - (3158-1275)×0.25
A
A
E
A
A
E
A
A
E
A
A
E
A
A
E
= +3862 - 471 = 3391 kJ
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.100
Find the heat of evaporation, hfg, for isobutane (Tc = 408.2 K, Pc = 3.65 MPa, M
= 58.124) at 12.6°C from the generalized charts and compare to the values in the
CATT3 computerized tables.
A
A
A
E
A
A
A
E
E
To read the charts we need the reduced temperature
Tr1 = T/Tc = (12.6 + 273.15)/408.2 = 0.70
A
A
A
A
E
E
(h*− h)g = 0.2 RTc ; (h*− h)f = 4.85 RTc
E
E
A
A
A
A
A
A
E
A
A
A
E
A
A
E
E
hfg = hg – hf = − (h*− h)g − (h*− h)f = (−0.2 + 4.85) RTc
E
A
A
E
A
A
E
A
A
A
E
E
A
A
A
A
E
A
A
A
A
E
E
= 4.65 RTc = 4.65 × (8.3145/58.124) × 408.2
A
A
E
= 271.5 kJ/kg
CATT3:
hfg = -25.62 + 368.7 = 343 kJ/kg
A
A
E
The generalized charts are not super accurate, some improvement can be done
using the accentric factor.
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.101
A cylinder contains ethylene, C2H4, at 1.536 MPa, −13°C. It is now compressed
isothermally in a reversible process to 5.12 MPa. Find the specific work and heat
transfer.
A
A
A
E
A
E
Ethylene C2H4 P1 = 1.536 MPa , T2 = T1 = -13oC = 260.2 K
E
A
A
A
A
E
A
E
A
A
A
E
A
A
E
A
A
E
Tr2 = Tr1 = 260.2 / 282.4 = 0.921 , Pr1 = 1.536 / 5.04 = 0.305
A
A
A
A
E
A
A
E
E
From D.1, D.2 and D.3:
Z1 = 0.85
A
A
E
*
(h1-h1) = 0.2964×282.4×0.40 = 33.5 and
A
A
A
A
E
E
From D.1, D.2 and D.3:
Z2 = 0.17 ,
A
A
A
A
E
A
A
A
E
E
A
E
and
A
E
A
Pr2 = 5.12/5.04 = 1.016 (comp. liquid)
A
*
(h2-h2) = 0.2964×282.4×4.0 = 334.8
A
*
(s1-s1) = 0.2964×0.30 = 0.0889
E
*
(s2-s2) = 0.2964×3.6 = 1.067
A
A
A
E
A
E
5.12
= -0.3568
1.536
1q2 = T(s2-s1) = 260.2(-1.067 - 0.3568 + 0.0889) = -347.3 kJ/kg
*
Ideal gas:
*
(h2-h1) = 0
A
A
A
* *
and
A
E
E
(s2-s1) = 0 - 0.2964 ln
A
A
A
E
A
A
A
E
E
A
A
A
A
E
A
A
E
A
A
E
E
(h2 - h1) = -334.8 + 0 + 33.5 = -301.3 kJ/kg
A
A
A
A
E
E
(u2 - u1) = (h2-h1) - RT(Z2-Z1) = -301.3 - 0.2964×260.2(0.17-0.85) = -248.9
A
A
A
A
E
A
E
A
A
E
A
A
E
A
E
A
A
E
1w2 = 1q2 - (u2 - u1) = -347.3 + 248.9 = -98.4 kJ/kg
A
A
A
E
A
A
E
A
A
E
A
A
E
A
A
E
A
E
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.102
Saturated vapor R-410A at 30°C is throttled to 200 kPa in a steady flow process.
Calculate the exit temperature assuming no changes in the kinetic energy, using
the generalized charts, Fig. D.2 and the R-410A tables, Table B.4.
R-410A throttling process
*
Energy Eq.:
*
*
*
h2 - h1 = 0 = (h2 - h2) + (h2 - h1) + (h1 - h1)
A
A
A
A
A
A
A
A
A
A
E
E
E
A
A
E
A
E
A
A
A
E
E
E
Generalized Chart, Fig. D.2, R = 8.31451/72.585 = 0.11455 kJ/kg-K
303.2
*
Tr1 = 344.5 = 0.88 => (h1-h1) = 0.11455 × 344.5 (0.85) = 33.54 kJ/kg
For CP0, use h values from Table B.4 at low pressure.
A
A
A
A
A
A
A
A
E
E
E
E
A
A
E
CP0 ≈ (330.83 - 314.40) / (40 - 20) = 0.8215 kJ/kg K
A
A
E
*
Substituting: (h2-h2) + 0.8215 (T2-30) + 33.54 = 0
A
A
A
A
A
A
E
E
E
at Pr2 = 200/4900 = 0.041
A
A
E
Assume T2 = -10oC => Tr2 = 263.2/344.5 = 0.764
E
A
A
A
A
A
A
E
E
*
(h2-h2) = RT × 0.1 = 0.11455 × 344.5 (0.1) = 3.95
A
A
A
A
E
E
Substituting :
-3.95 + 0.8215 (-10 - 30) + 33.54 = -3.27
Assume T2 = -5oC => Tr2 = 268.2/344.5 = 0.778
E
A
A
A
A
A
A
E
E
*
(h2-h2) = RT × 0.1 = 0.11455 × 344.5 (0.1) = 3.95
A
A
A
A
E
E
Substituting :
-3.95 + 0.8215 (-5 - 30) + 33.54 = 0.84
⇒ T2 = -6.0 oC
E
A
A
A
A
E
R-410A tables, B.4: at T1 = 30oC, x1 = 1.0
E
A
A
A
E
A
A
E
A
=> h1 = 284.16 kJ/kg
A
A
E
h2 = h1 = 284.16 , P2 = 0.2 MPa => T2 = -13.4 oC
E
A
A
E
A
A
E
A
A
E
A
A
A
A
E
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.103
Repeat Problem 12.91 using CATT3 and include the acentric factor for butane to
improve the accuracy.
C4H10 cycle
1
A
A
A
A
E
T1 = 80 oC, x1 = 1.0 ; T3 = 30oC, x3 = 0.0
353.2
Tr1 = 425.2 = 0.831
.
WT
Turbine
E
E
A
A
A
A
A
A
2
E
A
A
A
E
E
From CATT3 with ω = 0.199:
P1 = 0.2646 × 3800 = 1005 kPa
Cond
A
A
E
*
(h1-h1) = 0.1430 × 425.2×0.5685 = 34.6
*
(s1-s1) = 0.1430 × 0.4996 = 0.0714
P
4
A
A
A
E
Ht.
Exch
A
A
E
A
.
QH
E
A
E
A
A
A
A
E
E
.
-WP
3
A
A
A
A
E
E
303.2
Tr3 = 425.2 = 0.713
A
A
A
A
E
E
From CATT3 with ω = 0.199: P3 = 0.07443 × 3800 = 282.8 kPa
A
A
E
sat. liq.: (h - hf) = RTc×6.048 = 367.74 ;
(s*- sf) = R×8.399 = 1.201
sat. vap.: (h*- hg) = RTc×0.202 = 12.28 ;
(s*- sg) = R×0.201 = 0.0287
*
E
A
A
A
A
A
E
E
A
A
E
A
A
A
A
E
A
E
E
A
A
A
E
A
A
E
A
A
A
E
Because of the combination of properties of C4H10 (particularly the large CP0/R),
A
A
A
A
E
A
E
A
E
s1 is larger than sg at T3. To demonstrate,
A
A
A
A
A
A
E
E
E
353.2
1005
* *
(s1-sg3) = 1.716 ln 303.2 - 0.1430 ln 282.8 = 0.0806
A
A
A
A
A
E
A
A
A
E
E
E
(s1-sg3) = -0.0714 + 0.0806 + 0.0287 = +0.0379 kJ/kg K
A
A
A
A
E
E
T
1
so that T2S will be > T3, as shown in the T-s
diagram. A number of other heavy hydrocarbons
also exhibit this behavior.
A
A
E
A
3
2s 2
A
E
s
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
Solution 12.103 continued.
Assume T2S = 315 K,
=> Tr2S = 315 / 425.2 = 0.741
From CATT3:
*
(h2S - h2S) = RTc×0.183 = 11.13
and
*
(s2S - s2S) = R×0.1746 = 0.025
353.2
1005
*
*
(s1 - s2S) = 1.716 ln 315 - 0.1430 ln 282.8 = +0.01509
(s1 - s2S) = -0.0714 + 0.01509 + 0.025 = -0.031
Repeat at T2S = 310 K to get Tr2S = 0.729,
*
*
(h2S - h2S) = RTc×0.1907 = 11.595 and (s2S - s2S) = R×0.1853 = 0.0265
353.2
1005
*
*
(s1 - s2S) = 1.716 ln 310 - 0.1430 ln 282.8 = +0.04255
(s1 - s2S) = -0.0714 + 0.04255 + 0.0265 = -0.0023 very close to 0, OK
⇒ T2S = 310 K
*
*
(h1 - h2S) = 1.716 (353.2 - 310) = 74.13
wST = h1 - h2S = -34.6 + 74.13 + 11.6 = 51.1 kJ/kg
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.104
A cylinder contains ethylene, C2H4, at 1.536 MPa, −13°C. It is now compressed
in a reversible isobaric (constant P) process to saturated liquid. Find the specific
work and heat transfer.
Ethylene C2H4 ; P1 = 1.536 MPa = P2 ,
T1 = -13oC = 260.2 K
State 2: saturated liquid, x2 = 0.0
260.2
1.536
Tr1 = 282.4 = 0.921 Pr1 = Pr2 = 5.04 = 0.305
*
From Figs. D.1, D.2: Z1 = 0.85 , (h1 - h1)/RTc = 0.40
v1 =
Z1RT1
P1
=
0.85×0.29637×260.2
= 0.042675
1536
*
(h1 - h1) = 0.296 37 × 282.4 × 0.40 = 33.5
From Figs. D.1, D.2: T2 = 0.824×282.4 = 232.7 K
*
Z2 = 0.05 , (h2 - h2)/RTc = 4.42
v2 =
Z2RT2
P2
=
0.05×0.29637×232.7
= 0.002245 m3/kg
1536
*
(h2 - h2) = 0.296 37 × 282.4 × 4.42 = 369.9
*
*
(h2 - h1) = CP0(T2 - T1) = 1.5482(232.7 - 260.2) = -42.6
3
w12 = ⌠
⌡ Pdv = P(v2 - v1) = 1536 kPa (0.002 245 - 0.042 675) m /kg
= -62.1 kJ/kg
q12 = (u2 - u1) + w12 = (h2 - h1) = -369.9 - 42.6 + 33.5
= -379 kJ/kg
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.105
Refrigerant-123, dichlorotrifluoroethane, which is currently under development as
a potential replacement for environmentally hazardous refrigerants, undergoes an
isothermal steady flow process in which the R-123 enters a heat exchanger as
saturated liquid at 40°C and exits at 100 kPa. Calculate the heat transfer per
kilogram of R-123, using the generalized charts, Fig. D.2
R-123: M = 152.93, TC = 456.9 K, PC = 3.67 MPa
T1 = T2 = 40 oC, x1 = 0
P2 = 100 kPa
1
2
Heat
Tr1 = Tr2 = 313.2/456.9 = 0.685,
Pr2 = 0.1/3.67 = 0.027
From Fig. D.2: Pr1 = 0.084, (h* − h)1/RTC = 4.9
From D.1: saturated P1 = 0.084×3670 = 308 kPa
P2 < P1 with no work done, so process is irreversibel.
Energy Eq.:
q + h 1 = h 2,
From Fig. D.2:
Entropy Eq.:
s1 + ∫ dq/T + sgen = s2, sgen > 0
(h*- h)2/RTC = 0.056
q = h2 - h1 = 8.3145 × 456.9 [-0.056 + 0 + 4.90]/152.93 = 120.4 kJ/kg
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.106
Carbon dioxide collected from a fermentation process at 5°C, 100 kPa should be
brought to 243 K, 4 MPa in a steady flow process. Find the minimum amount of
work required and the heat transfer. What devices are needed to accomplish this
change of state?
278.2
100
Tri = 304.1 = 0.915, Pri = 7380 = 0.0136
From D.2 and D.3 : (h*-h) /RTC = 0.02, (s*-s)ri/R = 0.01
ri
243
Tre = 304.1 = 0.80,
From D.2 and D.3:
*
4
Pre = 7.38 = 0.542
(h*-h) /RTC = 4.5 ,
re
*
*
(s*-s)re/R = 4.74
*
(hi-he) = - (hi -hi) + (hi -he ) + (he -he)
= - 0.188 92×304.1×0.01 + 0.8418(278.2-243)
+ 0.188 92×304.1×4.5 = 287.6 kJ/kg
*
* *
*
(si-se) = - (si -si) + (si -se ) + (se -se)
= - 0.188 92×0.01 + 0.8418 ln(278.2/243)
- 0.188 92 ln(0.1/4) + 0.188 92×4.74 = 1.7044 kJ/kg K
wrev = (hi-he) -T0(si-se) = 287.6 - 278.2(1.7044) = -186.6 kJ/kg
qrev = (he-hi) + wrev = -287.6 -186.6 = -474.2 kJ/kg
We need a compressor to bring the pressure up and a cooler to bring the
temperature down. Cooling it before compression and intercooling between
stages in the compressor lowers the compressor work. In an actual set-up we
require more work than the above reversible limit.
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.107
Determine how accurate the generalized chart is for the carbon dioxide process in
Problem 12.106 by using the CATT3 software for the carbon dioxide properties.
From the CATT3 software:
Inlet superheated vapor
hi = 376.1 kJ/kg and
si = 2.015 kJ/kg-K
Exit compressed liquid
he = 20.21 kJ/kg and
se = 0.07349 kJ/kg-K
wrev = (hi – he) – T0(si – se) = 376.1 – 20.21 – 278.2 (2.015 – 0.07349)
= -184.1 kJ/kg
q
rev
= (he – hi) + wrev = (20.21 – 376.1) – 184.1 = -540 kJ/kg
The work is very accurate but the heat transfer a little less so. The enthalpy and
entropy differences are both under-estimated by the generalized charts.
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.108
A geothermal power plant on the Raft River uses isobutane as the working fluid.
The fluid enters the reversible adiabatic turbine at 160°C, 5.475 MPa, and the
condenser exit condition is saturated liquid at 33°C. Isobutane has the properties
Tc= 408.14 K, Pc= 3.65 MPa, CP0= 1.664 kJ/kg K and ratio of specific heats k =
1.094 with a molecular weight as 58.124. Find the specific turbine work and the
specific pump work.
Turbine inlet: T1 = 160oC , P1 = 5.475 MPa
Condenser exit: T3 = 33oC , x3 = 0.0, Tr3 = 306.2 / 408.1 = 0.75
From Fig. D.1:
Pr3 = 0.16, Z3 = 0.03
=> P2 = P3 = 0.16 × 3.65 = 0.584 MPa
Tr1 = 433.2 / 408.1 = 1.061,
Pr1 = 5.475 / 3.65 = 1.50
From Fig. D.2 & D.3:
*
(h1 - h1) = 0.143 05×408.1×2.84 = 165.8
*
(s1 - s1) = 0.143 05×2.15 = 0.3076
0.584
306.2
*
*
(s2 - s1) = 1.664 ln 433.2 - 0.143 05 ln 5.475 = -0.2572
*
*
(s2 - s2) = (s2 - sF2) - x2 sFG2
= 0.143 05×6.12 - x2×0.143 05(6.12-0.29) = 0.8755 - x2×0.8340
(s2 - s1) = 0 = -0.8755 + x2×0.8340 - 0.2572 + 0.3076 => x2 = 0.99
*
*
(h2 - h1) = CP0(T2 - T1) = 1.664(306.2 - 433.2) = -211.3
From Fig. D.2:,
*
*
(h2 - h2) = (h2 - hF2) - x2hFG2 = 0.143 05×408.1[4.69-0.99(4.69-0.32)]
Turbine:
= 273.8 − 0.99 × 255.1 = 21.3
wT = (h1 - h2) = -165.8 + 211.3 + 21.3 = 66.8 kJ/kg
Pump:
vF3 =
ZF3RT3 0.03×0.143 05×306.2
= 0.00225
P3 =
584
wP = - ∫ v dP ≈ vF3(P4 - P3) = -0.00225 (5475-584) = -11.0 kJ/kg
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.109
A flow of oxygen at 230 K, 5 MPa is throttled to 100 kPa in a steady flow
process. Find the exit temperature and the entropy generation.
Process: Throttling
.
Small surface area: Q = 0;
.
No shaft:
W=0
.
Irreversible:
Sgen > 0
We will solve the problem using generalized charts.
230
5
0.1
Tri = 154.6 = 1.488, Pri = 5.04 = 0.992, Pre = 5.04 = 0.02
From D.2:
Energy Eq.:
*
(hi -hi) = RTc ∆h = 0.2598 × 154.6 × 0.50 = 20.1 kJ/kg
*
*
*
*
(he- hi) = 0 = - (he -he) + (he -hi ) + (hi -hi)
Assume Te = 208 K , Tre = 1.345:
*
*
(he -hi ) = Cp (Te – Ti) = 0.922 (208 - 230) = -20.3 kJ/kg
From D.2:
*
(he -he) = RTc ∆h = 0.2598 × 154.6 × 0.01 = 0.4
Check first law (he- hi) = -0.4 -20.3 + 20.1 ≈ 0 OK => Te = 208 K
From D.3,
*
(si -si) = 0.2598×0.25 = 0.0649
and
*
(se -se) = 0.2598×0.01 = 0.0026
0.1
208
* *
(se -si ) = 0.9216 ln 230 - 0.2598 ln 5 = 0.9238 kJ/kg K
sgen = (se- si) = -0.0026 + 0.9238 + 0.0649 = 0.9861 kJ/kg K
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.110
An uninsulated piston/cylinder contains propene, C3H6, at ambient temperature,
19°C, with a quality of 50% and a volume of 10 L. The propene now expands
very slowly until the pressure in the cylinder drops to 460 kPa. Calculate the mass
of propene, the work, and heat transfer for this process.
T1 = 19oC = 292.2 K,
Propene C3H6:
x1 = 0.50,
From Fig. D.1:
Tr1 = 292.2/364.9 = 0.80,
Pr1 = Pr sat = 0.25,
P1 = 0.25 × 4.6 = 1.15 MPa
From D.1:
Z1 = 0.5 × 0.04 + 0.5 × 0.805 = 0.4225
P1V1
m = Z RT =
1150×0.010
= 0.471 kg
0.4225×0.197 58×292.2
1
1
V1 = 10 L
Assume reversible and isothermal process (slow, no friction, not insulated)
Q = m(u2-u1) + 1W2
1 2
2
2
W = ⌠ PdV (cannot integrate);
1 2 ⌡
Q = ⌠ TdS = Tm(s2-s1)
1 2 ⌡
1
1
From Figs. D.2 and D.3:
*
h1 - h1 = 0.19758 × 364.9(0.5 × 4.51 + 0.5 × 0.46) = 179.2 kJ/kg
*
(s1 - s1) = 0.197 58 (0.5 × 5.46 + 0.5 × 0.39) = 0.5779 kJ/kg K
The ideal gas change in h and s are
*
*
(h2 - h1) = 0 and
460
*
*
(s2 - s1) = 0 - 0.197 58 ln 1161 = + 0.1829 kJ/kg K
At Tr2 = 0.80, Pr2 = 0.10, from D.1, D.2 and D.3, Z2 = 0.93
*
(h2 - h2) = 0.197 58 × 364.9 × 0.16 = 11.5 kJ/kg
*
(s2 - s2) = 0.197 58 × 0.13 = 0.0257 kJ/kg K
Now we can do the change in s and h from state 1 to state 2
*
*
*
*
(s2 - s1) = -(s2 - s2) + (s2 - s1) + (s1 - s1)
= -0.0257 + 0.1829 + 0.5779 = 0.7351 kJ/kg K
*
*
*
*
(h2 - h1) = - (h2 - h2) + (h2 - h1) + h1 - h1
= -11.5 + 0 + 179.2 = 167.7 kJ/kg
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
Solution 12.110 continued.
The heat transfer is found from the second law
q = 292.2 × 0.7351 = 214.8 kJ/kg
1 2
=>
Q = m 1q2 = 101.2 kJ
1 2
We need the internal energy in the energy equation
u2 - u1 = (h2 - h1) + RT(Z1 - Z2) = 167.7 + 0.197 58 × 292.2 (0.4225 - 0.93)
= 138.4 kJ/kg
w = 1q2 - (u2 - u1) = 214.8 - 138.4 = 76.4 kJ/kg
1 2
1
W2 = m 1w2 = 36.0 kJ
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.111
An alternative energy power plant has carbon dioxide at 6 MPa, 100oC flowing
into a turbine with an exit as saturated vapor at 1 MPa. Find the specific turbine
work using generalized charts and repeat using Table B.3.
From Table A.5:
R = 0.1889 kJ/kgK, CP = 0.842 kJ/kg
From Table A.2:
TC = 304.1 K,
6
Pri = 7.38 = 0.813,
PC = 7.38 MPa
373.2
Tri = 304.1 = 1.227
~
Δ h = 0.70
From D.2 and D.3,
~
*
(hi - hi) = RTC × Δ h = 0.1889 × 304.1 × 0.70 = 46.8 kJ/kg;
1
Pre = 7.38 = 0.1355, x = 1 so Tre = 0.73, Te = 0.73 ×304.1 = 222 K
From D.2 and D.3,
~
*
(he - he) = RTC × Δ h = 0.1889 × 304.1 × 0.25 = 46.8 kJ/kg;
*
*
*
*
w = hi – he = hi – he – (hi - hi) + (he - he)
~
~
= CP( hi – he) – RTC (Δ hi – Δ he)
= 0.842 (373.15 – 222) – 0.1889 × 0304.1 (0.7 – 0.25)
= 101.45 kJ/kg
From Table B.3
w = hi – he = 421.69 – 322.39 = 99.3 kJ/kg
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.112
A distributor of bottled propane, C3H8, needs to bring propane from 350 K, 100 kPa
to saturated liquid at 290 K in a steady flow process. If this should be accomplished
in a reversible setup given the surroundings at 300 K, find the ratio of the volume
. .
flow rates Vin/Vout, the heat transfer and the work involved in the process.
350
From Table A.2: Tri = 369.8 = 0.946 ,
0.1
Pri = 4.25 = 0.024
From D.1, D.2 and D.3,
Zi = 0.99
*
(hi -hi) = 0.1886 × 369.8 × 0.03 = 2.1 kJ/kg
*
(si -si) = 0.1886 × 0.02 = 0.0038 kJ/kg K
290
369.8 = 0.784, and x = 0
From D.1, D.2 and D.3,
Pre = 0.22 , Pe = 0.22 × 4.25 = 0.935 MPa and
Tre =
Ze = 0.036
*
(he -he) = 0.1886 × 369.8 × 4.57 = 318.6 kJ/kg
*
(se -se) = 0.1886 × 5.66 = 1.0672 kJ/kg K
*
*
(he -hi ) = 1.679(290 - 350) = -100.8 kJ/kg
0.935
290
* *
(se -si ) = 1.679 ln 350 - 0.1886 ln 0.1 = -0.7373 kJ/kg K
(he-hi) = -318.6 - 100.8 + 2.1 = -417.3 kJ/kg
(se-si) = -1.0672 - 0.7373 + 0.0038 = -1.8007 kJ/kg K
.
Vin
.
Vout
ZiTi/Pi
0.99 350 0.935
= Z T /P = 0.036 × 290 × 0.1 = 310.3
e e
e
wrev = (hi-he) -T0(si-se) = 417.3 - 300(1.8007) = -122.9 kJ/kg
qrev = (he-hi) + wrev = -417.3 –122.9 = -540.2 kJ/kg
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.113
An insulated cylinder fitted with a frictionless piston contains saturated-vapor
carbon dioxide at 0oC, at which point the cylinder volume is 20 L. The external
force on the piston is now slowly decreased, allowing the carbon dioxide to
expand until the temperature reaches - 30oC. Calculate the work done by the
CO2 during this process.
CO2: TC = 304.1 K, Pc = 7.38 MPa, Cp = 0.842 kJ/kg-K, R = 0.1889 kJ/kg K
State 1: T1 = 0oC, sat. vap., x1 = 1.0, V1 = 20 L
Tr1 = 0.9, P1 = Pr1PC = 0.53 × 7380 = 3911 kPa, Z1 = Zg = 0.67
P V
(h*1 − h1)g = 0.9 RTC, (s*1 − s1)g/R = 0.72, m = Z 1RT1 = 2.262 kg
1
1
State 2: T2 = -30oC
Tr2 = 0.8, P2 = Pr2Pc = 0.25 × 7380 = 1845 kPa
Entropy Eq.:
Sgen = m(s2 − s1) − 1Q2/T ;
1Q2 = 0,
*
*
*
Sgen = 0
*
s2 - s1 = (s2 − s2) + (s2 − s1) + (s1 − s1) = 0
T2
P2
*
*
*
s2 − s1 = CP ln T − R ln P = 0.044 kJ/kg-K, s1 − s1 = 0.136 kJ/kg-K
1
1
*
s2 - s2 = 0.180 kJ/kg K,
(s*2 − s2)f = 5.46 R, (s*2 − s2)g = 0.39 R
(s*2 − s2) = (1-x2)(s*2 − s2)f + x2 (s*2 − s2)g x2 = 0.889
Energy Eq.:
1Q2 = m(u2 − u1) + 1W2 ; 1Q2 = 0,
u = h - Pv
Z2 = (1 - x2)Zf + x2Zg = 0.111 × 0.04 + 0.889 × 0.81 = 0.725;
*
*
*
*
(h2 - h1) = (h2 − h2) + (h2 − h1) + (h1 − h1)
*
*
*
h2 − h1 = Cp(T2 - T1) = -25.3 kJ/kg, (h1 − h1) = 51.7 kJ/kg
(h*2 − h2)f = 4.51 RTC , (h*2 − h2)g = 0.46 RTC
(h*2 − h2) = (1 - x2)(h*2 − h2)f + x2 (h*2 − h2)g = 52.2 kJ/kg
h2 - h1 = -52.2 – 25.3 + 51.7 = -25.8 kJ/kg
u2 - u1 = (h2 - h1) - Z2RT2 + Z1RT1 = -25.8 – 0.725 × 0.18892 × 243.2
+ 0.67 × 0.18892 × 273.2 = -24.5 kJ/kg
1W2 = 55.4 kJ
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.114
A control mass of 10 kg butane gas initially at 80°C, 500 kPa, is compressed in a
reversible isothermal process to one-fifth of its initial volume. What is the heat
transfer in the process?
Butane C4H10: m = 10 kg,
T1 = 80 oC, P1 = 500 kPa
Compressed, reversible T = const, to V2 = V1/5
353.2
500
Tr1 = 425.2 = 0.831, Pr1 = 3800 = 0.132
Z1 = 0.92,
From D.1 and D.3:
v1 =
Z1RT1
P1
=
*
(s1- s1) = 0.143×0.16 = 0.0230
0.92×0.143×353.2
= 0.09296 m3/kg
500
v2 = v1/5 = 0.01859 m3/kg;
State 2:
Tr2 = Tr1 = 0.831
From D.1: PG = 0.325×3800 = 1235 kPa
(s*-sF) = R×5.08 = 0.7266
sat. liq.: ZF = 0.05,
sat. vap.: ZG = 0.775,
(s*-sG) = R×0.475 = 0.0680
Therefore
vF =
0.05×0.143×353.2
= 0.00205 m3/kg
1235
vG =
0.775×0.143×353.2
= 0.0317 m3/kg
1235
Since vF < v2 < vG → x2 = (v2-vF)/(vG-vF) = 0.5578
*
*
*
(s2 - s2) = (1 - x2)(s2 - sF2) + x2(s2 - sG2)
= 0.4422 × 0.7266 + 0.5578 × 0.0680 = 0.3592 kJ/kg K
1235
*
*
(s2 - s1) = CP0 ln (T2/T1) - R ln (P2/P1) = 0 - 0.143 ln 500 = -0.1293
(s2 - s1) = -0.3592 - 0.1293 + 0.0230 = -0.4655 kJ/kg K
Q = Tm(s2 - s1) = 353.2 × 10 (-0.4655) = -1644 kJ
1 2
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.115
A line with a steady supply of octane, C8H18, is at 400°C, 3 MPa. What is your
best estimate for the availability in a steady flow setup where changes in potential
and kinetic energies may be neglected?
Ti = 400 oC, Pi = 3 MPa
Availability of Octane at
From Table A.5:
R = 0.07279 kJ/kgK, CP = 1.711 kJ/kg
From Table A.2:
TC = 568.8 K,
3
Pri = 2.49 = 1.205,
PC = 2.49 MPa
673.2
Tri = 568.8 = 1.184
From D.2 and D.3,
~
*
(hi - hi) = RTC × Δh = 0.072 79 × 568.8 × 1.13 = 46.8 kJ/kg;
*
~
(si - si) = R Δs = 0.072 79 × 0.69 = 0.05 kJ/kgK
This is relative to the dead ambient state, assume T0 = 298.2 K, P0 = 100 kPa
298.2
Tr0 = 568.8 = 0.524 ,
0.1
Pr0 = 2.49 = 0.040
From D.2 and D.3, The s correction is outside chart (extrapolate or use CATT3)
*
(h0 - h0) = RTC × 5.4 = 223.6
*
and
*
(s0 - s0) = R × 9 = 0.655 kJ/kgK
*
(hi - h0) = CP(Ti – T0) = 1.711 (673.2 – 298.2) = 641.7 kJ/kg
673.2
3
*
*
(si - s0) = 1.711 × ln 298.2 – 0.072 79 × ln 0.1 = 1.1459 kJ/kgK
(hi - h0) = -46.8 + 641.7 + 223.6 = 818.5 kJ/kg
(si - s0) = -0.05 + 1.1459 + 0.655 = 1.7509 kJ/kgK
ϕi = wrev = (hi - h0) - T0(si - s0) = 818.5 – 298.2(1.7509) = 296.5 kJ/kg
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.116
The environmentally safe refrigerant R-152a is to be evaluated as the working
fluid for a heat pump system that will heat a house. It uses an evaporator
temperature of –20oC and a condensing temperature of 30oC. Assume all
processes are ideal and R-152a has a heat capacity of Cp = 0.996 kJ/kg K.
Determine the cycle coefficient of performance.
Ideal Heat Pump TH = 30 oC
From A.2:
T
M = 66.05, R = 0.125 88, TC = 386.4 K, PC = 4.52 MPa
303.2
Tr3 = 386.4 = 0.785
2
3
4
Pr3 = Pr2 = 0.22
1
Sat.liq.:
=> P3 = P2 = 994 kPa
*
h3 - h3 = 4.56×RTC = 221.8
v
T1 = -20 oC = 253.2 K, Tr1 = 0.655, Pr1 = 0.058 → P1 = 262 kPa
*
h1 - h1 = 0.14×RTC = 6.8
and
*
s1 - s1 = 0.14×R = 0.0176
Assume T2 = 307 K, Tr2 = 0.795 given Pr2 = 0.22
*
From D.2, D.3: s2 - s2 = 0.34×R = 0.0428 ;
*
h2 - h2 = 0.40×RTc = 19.5
307
994
*
*
s2 - s1 = 0.996 ln 253.2 - 0.125 88 ln 262 = 0.0241
s2 - s1 = -0.0428 + 0.0241 + 0.0176 = -0.001 ≈ 0 OK
⇒ h2 - h1 = -19.5 + 0.996(307-253.2) + 6.8 = 40.9
h2 - h3 = -19.5 + 0.996(307-303.2) + 221.8 = 206.1
qH h2 - h3 206.1
β = w = h - h = 40.9 = 5.04
IN
2
1
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.117
Rework the previous problem using an evaporator temperature of 0oC.
Ideal Heat Pump TH = 30 oC
From A.2:
T
M = 66.05, R = 0.125 88, TC = 386.4 K, PC = 4.52 MPa
303.2
Tr3 = 386.4 = 0.785
2
3
1
4
Pr3 = Pr2 = 0.22
Sat.liq.:
=> P3 = P2 = 994 kPa
*
h3 - h3 = 4.56×RTC = 221.8
v
T1 = 0 oC = 273.2 K, Tr1 = 0.707 => Pr1 = 0.106, P1 = 479 kPa
*
h1 - h1 = 0.22×RTC = 10.7
*
and
s1 - s1 = 0.21×R = 0.0264
and
h2 - h2 = 0.38×RTC = 18.5
Assume T2 = 305 K, Tr2 = 0.789
*
s2 - s2 = 0.35×R = 0.0441
*
305.0
994
*
*
s2 - s1 = 0.996 ln 273.2 - 0.125 88 ln 479 = 0.0178
s2 - s1 = -0.0441 + 0.0178 + 0.0264 = 0.0001 ≈ 0 OK
h2 - h1 = -18.5 + 0.996(305.0-273.2) + 10.7 = 23.9
h2 - h3 = -18.5 + 0.996(305.0-303.2) + 221.8 = 205.1
h2 - h3 205.1
β = h - h = 23.9 = 8.58
2
1
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.118
An uninsulated compressor delivers ethylene, C2H4, to a pipe, D = 10 cm, at 10.24
MPa, 94°C and velocity 30 m/s. The ethylene enters the compressor at 6.4 MPa,
20.5°C and the work input required is 300 kJ/kg. Find the mass flow rate, the total
heat transfer and entropy generation, assuming the surroundings are at 25°C.
293.7
6.4
Tri = 282.4 = 1.040 , Pri = 5.04 = 1.270
From D.2 and D.3,
*
(hi -hi) = 0.296 37 × 282.4 × 2.65 = 221.8 kJ/kg
*
(si -si) = 0.296 37 × 2.08 = 0.6164 kJ/kg K
367.2
10.24
Tre = 282.4 = 1.30 , Pre = 5.04 = 2.032 => From D.1:
ve =
Ae =
ZeRTe
Pe
=
Ze = 0.69
0.69×0.296 37×367.2
= 0.0073 m3/kg
10 240
π 2
D = 0.007 85 m2
4 e
=>
. AeVe 0.007 85×30
m = v = 0.0073 = 32.26 kg/s
e
From D.2 and D.3,
*
(he -he) = 0.296 37 × 282.4 × 1.6 = 133.9 kJ/kg
*
(se -se) = 0.296 37 × 0.90 = 0.2667 kJ/kg K
*
*
(he -hi ) = 1.5482(367.2-293.7) = 113.8
10.24
367.2
* *
(se -si ) = 1.5482 ln 293.7 - 0.296 37 ln 6.4 = 0.2065
(he-hi) = -133.9 + 113.8 + 221.8 = 201.7 kJ/kg
(se-si) = -0.2667 + 0.2065 + 0.6164 = 0.5562 kJ/kg K
Energy Eq.:
q = (he-hi) + KEe + w = 201.7 +
302
- 300 = -97.9 kJ/kg
2×1000
.
.
Qcv = mq = 32.26(-97.9) = -3158 kW
.
Qcv .
.
3158
Sgen = − T + m(se - si) = + 298.2 + 32.26(0.5562) = 28.53 kW/K
o
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.119
The new refrigerant fluid R-123 (see Table A.2) is used in a refrigeration system
that operates in the ideal refrigeration cycle, except the compressor is neither
reversible nor adiabatic. Saturated vapor at -26.5°C enters the compressor and
superheated vapor exits at 65°C. Heat is rejected from the compressor as 1 kW,
and the R-123 flow rate is 0.1 kg/s. Saturated liquid exits the condenser at 37.5°C.
Specific heat for R-123 is CP = 0.6 kJ/kg. Find the coefficient of performance.
R-123: Tc = 456.9 K, Pc = 3.67 MPa, M = 152.93 kg/kmol, R = 0.05438 kJ/kg K
State 1: T1 = -26.5oC = 246.7 K, sat vap., x1 = 1.0
Tr1 = 0.54, Fig D.1, Pr1 = 0.01, P1 = Pr1Pc = 37 kPa
Fig. D.2,
*
h1-h1 = 0.03 RTC = 0.8 kJ/kg
State 2: T2 = 65oC = 338.2 K
State 3: T3 = 37.5oC = 310.7 K, sat. liq., x3 = 0
Tr3 = 0.68, Fig. D.1: Pr3 = 0.08, P3 = Pr3Pc = 294 kPa
P2 = P3 = 294 kPa, Pr2 = 0.080, Tr2 = 0.74,
*
h2-h2 = 0.25 RTC = 6.2 kJ/kg
Fig. D.2:
*
h3-h3 = 4.92 RTC = 122.2 kJ/kg
State 4: T4 = T1 = 246.7 K,
h4 = h3
Energy Eq. Evaporator: qL + h4 = h1 + w; w = 0,
*
*
*
h4 = h3
*
qL = h1 - h3 = (h1 − h1) + (h1 − h3) + (h3 − h3)
*
*
h1 − h3 = CP(T1 - T3) = -38.4 kJ/kg, qL = -0.8 – 38.4 + 122.2 = 83.0 kJ/kg
Energy Eq. Compressor: q + h1 = h2 + wc;
.
Q = -1.0 kW,
*
*
*
.
m = 0.1 kg/s
*
wc = h1 - h2 + q; h1 - h2 = (h1 − h1) + (h1 − h2) + (h2 − h2)
*
*
h1 − h2 = CP(T1 - T2) = -54.9 kJ/kg,
wc = -0.8 –54.9 + 6.2 – 10.0 = -59.5 kJ/kg
β = qL/wc = 83.0/59.5 = 1.395
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.120
An evacuated 100-L rigid tank is connected to a line flowing R-142b gas,
chlorodifluoroethane, at 2 MPa, 100°C. The valve is opened, allowing the gas to
flow into the tank for a period of time, and then it is closed. Eventually, the tank
cools to ambient temperature, 20°C, at which point it contains 50% liquid, 50%
vapor, by volume. Calculate the quality at the final state and the heat transfer for
the process. The ideal-gas specific heat of R-142b is Cp = 0.787 kJ/kg K.
Rigid tank V = 100 L, m1 = 0
Line: R-142b CH3CClF2
M = 100.495, TC = 410.3 K, PC = 4.25 MPa,
CP0 = 0.787 kJ/kg K
−
R = R/M = 8.31451 / 100.495 = 0.082 73 kJ/kg K
Line Pi = 2 MPa, Ti = 100 oC, Flow in to T2 = T0 = 20oC
VLIQ 2 = VVAP 2 = 50 L
Continuity: mi = m2 ; Energy:
QCV + mihi = m2u2 = m2h2 - P2V
From D.2 at i: Pri = 2 / 4.25 = 0.471,
Tri = 373.15 / 410.3 = 0.91
*
(hi -hi) = 0.082 73×410.3×0.72 = 24.4
*
*
(h2-hi ) = CP0(T2-Ti) = 0.787(20-100) = -63.0
293.2
From D.2: Tr2 = 410.3 = 0.715 => P2 = 0.115×4250 = 489 kPa
sat. liq.:
ZF = 0.02, (h*-hF) = RTC×4.85 = 164.6
sat. vap.:
ZG = 0.88, (h*-hG) = RTC×0.25 = 8.5
P2VLIQ 2
mLIQ 2 = Z RT =
F
2
489×0.050
= 50.4 kg
0.02×0.082 73×293.2
P2VVAP 2
mVAP 2 = Z RT = 1.15 kg,
G
2
m2 = 51.55 kg
x2 = mVAP 2/m2 = 0.0223
*
*
*
(h2-h2) = (1-x2)(h2-hF2) + x2(h2-hG2) = 0.9777 × 164.6 + 0.0223 × 8.5 = 161.1
QCV = m2(h2 - hi) - P2V = 51.55(-161.1 - 63.0 + 24.4) – 489 × 0.10
= -10 343 kJ
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
Mixtures
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.121
A 2 kg mixture of 50% argon and 50% nitrogen by mole is in a tank at 2 MPa,
180 K. How large is the volume using a model of (a) ideal gas and (b) Kays
rule with generalized compressibility charts.
a) Ideal gas mixture
Eq.11.5:
Mmix = ∑ yi Mi = 0.5 × 39.948 + 0.5 × 28.013 = 33.981
−
mRT 2 × 8.3145 × 180
V=M P=
= 0.044 m3
33.981 × 2000
mix
b) Kay’s rule Eq.12.84
Pc mix = 0.5 × 4.87 + 0.5 × 3.39 = 4.13 MPa
Tc mix = 0.5 × 150.8 + 0.5 × 126.2 = 138.5 K
2
180
Reduced properties:
Pr = 4.13 = 0.484, Tr = 138.5 = 1.30
Fig. D.1: Z = 0.925
−
mRT
V = Z M P = 0.925 × 0.044 = 0.0407 m3
mix
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.122
A 2 kg mixture of 50% argon and 50% nitrogen by mass is in a tank at 2 MPa,
180 K. How large is the volume using a model of (a) ideal gas and (b) van der
Waals equation of state with a, b for a mixture?
a) Ideal gas mixture
Eq.11.15: Rmix = ∑ ci Ri = 0.5 × 0.2081 + 0.5 × 0.2968 = 0.25245 kJ/kg K
mRmixT 2 × 0.25245 × 180
V=
=
= 0.0454 m3
P
2000
b) van der Waals equation of state. before we can do the parameters a, b for the
mixture we need the individual component parameters.
2 2
27 R Tc 27 (0.2081 × 150.8)2
aAr = 64 P = 64
= 0.08531
4870
c
A
A
A
E
E
E
2 2
27 R Tc 27 (0.2968 × 126.2)2
aN2 = 64 P = 64
= 0.17459
3390
c
RTc 0.2081 × 150.8
bAr = 8P =
= 0.000 805
8 × 4870
c
RTc 0.2968 × 126.2
bN2 = 8P =
= 0.001 381
8 × 3390
c
Now the mixture parameters are from Eq.12.84
AE
A
A
A
E
E
A
A
A
A
E
E
E
A
A
E
A
A
E
amix =
A
2
∑ ci a1/2
i = (0.5 × 0.08531 + 0.5 × 0.17459) = 0.126
2
E
E AE
A
EA
A
EA
A
A
bmix = ∑ ci bi = 0.5 × 0.000 805 + 0.5 × 0.001 381 = 0.001 093
RT
a
Using now Eq.12.52:
P=
− 2
v−b v
0.25245 × 180 0.126
2000 =
− 2
v − 0.001 093
v
By trial and error we find the specific volume, v = 0.02097 m3/kg
V = mv = 0.04194 m3
A
A
E
A
A
E
A
A
E
A
E
E
A
A
E
A
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.123
R-410A is a 1:1 mass ratio mixture of R-32 and R-125. Find the specific volume
at 20oC, 1200 kPa using Kays rule and the generalized charts and compare to
Table B.4
E
A
A
Kay’s rule Eq.12.83
Pc mix = 0.5 × 5.78 + 0.5 × 3.62 = 4.70 MPa
Tc mix = 0.5 × 351.3 + 0.5 × 339.2 = 345.25 K
1.2
293.15
Reduced properties: Pr = 4.70 = 0.255, Tr = 345.25 = 0.849
Table A.5: R = 0.1145 kJ/kg-K or compute from mix
Fig. D.1: Z = 0.85
v = ZRT/P = 0.85 × 0.1145 × 293.15 / 1200 = 0.0238 m3/kg
Table B.4: v = 0.02260 m3/kg
A
A
A
E
A
E
E
A
A
E
A
A
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.124
The R-410A in Problem 12.123 is flowing through a heat exchanger with an
exit at 120oC, 1200 kPa. Find the specific heat transfer using Kays rule and the
generalized charts and compare to solution using Table B.4
E
A
A
Rmix = 0.5 × 0.1598 + 0.5 × 0.06927 = 0.1145 kJ/kg-K,
CP mix = ∑ ci CP i = 0.5 × 0.822 + 0.5 × 0.791 = 0.8065 kJ/kg K
Kay’s rule Eq.12.84
Pc mix = 0.5 × 5.78 + 0.5 × 3.62 = 4.70 MPa
Tc mix = 0.5 × 351.3 + 0.5 × 339.2 = 345.25 K
1.2
293.15
Reduced properties 1:
Pr1 = 4.70 = 0.255, Tr1 = 345.25 = 0.849
A
A
A
A
E
E
(h*1 − h1) = 0.4 × RTc = 0.4 × 0.1145 × 345.25 = 15.81 kJ/kg
Fig. D.1:
A
A
A
A
A
A
E
E
E
1.2
Pr2 = 4.70 = 0.255,
Reduced properties 1:
A
A
E
*
h2 − h2
(
Fig. D.1:
A
A
A
A
E
) = 0.2 × RTc = 0.2 × 0.1145 × 345.25 = 7.906 kJ/kg
A
E
393.15
Tr2 = 345.25 = 1.139
A
A
A
E
E
The energy equation gives
1q2 = (h2 - h1) =
A
A
E
A
A
A
E
A
E
A
A
E
(h2 − h*2) + (h*2 − h*1) + (h*1 − h1)
A
A
A
E
A
A
E
A
A
E
A
A
E
A
E
A
A
E
= -7.906 + 0.8065 (120 – 20) + 15.81
= 88.55 kJ/kg mix
Table B.4.2:
q = h2 – h1 = 393.13 – 290.51 = 102.62 kJ/kg
A
A
E
A
A
E
The main difference is in the value of specific heat, about 1 kJ/kg-K at the avg.
T, whereas it is 0.8 kJ/kg-K at 25oC.
E
A
A
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.125
A 2 kg mixture of 50% argon and 50% nitrogen by mass is in a tank at 2 MPa,
180 K. How large is the volume using a model of (a) ideal gas and (b) Redlich
Kwong equation of state with a, b for a mixture.
a) Ideal gas mixture
Eq.11.15: Rmix = ∑ ci Ri = 0.5 × 0.2081 + 0.5 × 0.2968 = 0.25245 kJ/kg K
mRmixT 2 × 0.25245 × 180
V=
=
= 0.0454 m3
P
2000
b) Redlich Kwong equation of state. Before we can do the parameters a, b for the
mixture we need the individual component parameters, Eq.12.54, 13.55.
E
A
A
A
E
5/2
R2Tc
0.20812 × 150.82.5
aAr = 0.42748 P
= 0.42748
= 1.06154
4870
c
AE
A
E
A
A
E
E
5/2
R2Tc
0.29682 × 126.22.5
aN2 = 0.42748 P
= 0.42748
= 1.98743
3390
c
RTc
0.2081 × 150.8
bAr = 0.08664 P = 0.08664
= 0.000 558
4870
c
RTc
0.2968 × 126.2
bN2 = 0.08664 P = 0.08664
= 0.000 957
3390
c
Now the mixture parameters are from Eq.12.84
AE
A
E
A
A
E
E
A
A
E
A
A
E
amix =
A
2
∑ ci a1/2
i = (0.5 × 1.06154 + 0.5 × 1.98743) = 1.4885
2
E
E AE
A
EA
A
EA
A
A
bmix = ∑ ci bi = 0.5 × 0.000 558 + 0.5 × 0.000 957 = 0.000 758
RT
a
Using now Eq.12.53:
P=
−
v − b v(v + b)T1/2
0.25245 × 180
1.4885
2000 =
−
v − 0.000 758 v(v + 0.000 758) 1801/2
By trial and error we find the specific volume, v = 0.02102 m3/kg
V = mv = 0.04204 m3
A
A
A
A
E
A
E
A
A
E
E
E
A
A
E
A
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.126
A modern jet engine operates so that the fuel is sprayed into air at a P, T higher
than the fuel critical point. Assume we have a rich mixture of 50% n-octane and
50% air by mole at 500 K and 3.5 MPa near the nozzle exit. Do I need to treat this
as a real gas mixture or is an ideal gas assumption reasonable? To answer find Z
and the enthalpy departure for the mixture assuming Kay’s rule and the
generalized charts.
The mole fractions are:
yC8H18 = 0.5, yN2 = 0.5 × 0.79 = 0.395, yO2 = 0.5 × 0.21 = 0.105
Eq.11.5:
Mmix = ∑ yi Mi = 0.5 × 114.232 + 0.395 × 28.013 + 0.105 × 31.999
= 71.541
Kay’s rule Eq.12.84
Pc mix = 0.5 × 2.49 + 0.395 × 3.39 + 0.105 × 5.04 = 3.113 MPa
Tc mix = 0.5 × 568.8 + 0.395 × 126.2 + 0.105 × 154.6 = 350.5 K
3.5
500
Reduced properties:
Pr = 3.113 = 1.124, Tr = 350.5 = 1.427
Fig. D.1: Z = 0.87
I must treat it as a real gas mixture.
8.3145
Fig. D.2 h* − h = 0.70 × RTc = 0.70 × 71.541 × 350.5 = 28.51 kJ/kg
A
A
A
E
A
E
E
A
A
A
A
E
A
A
E
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.127
A mixture of 60% ethylene and 40% acetylene by moles is at 6 MPa, 300 K. The
mixture flows through a preheater where it is heated to 400 K at constant P. Using
the Redlich Kwong equation of state with a, b for a mixture find the inlet specific
volume. Repeat using Kays rule and the generalized charts.
To do the EOS we need the gas constant, so from Eq.11.5 we get
Mmix = ∑ yi Mi = 0.6 × 28.054 + 0.4 × 26.068 = 27.26
Rmix = 8.3145/27.26 = 0.305 kJ/kg K
Redlich Kwong EOS the individual component parameters, Eq.12.54, 12.55.
5/2
R2Tc
0.29642 × 282.42.5
aC2H4 = 0.42748 P
= 0.42748
= 9.9863
5040
c
AE
A
E
A
A
E
E
5/2
R2Tc
0.31932 × 308.32.5
aC2H2 = 0.42748 P
= 0.42748
= 11.8462
6140
c
RTc
0.2964 × 282.4
bC2H4 = 0.08664 P = 0.08664
= 0.001 439
5040
c
RTc
0.3193 × 308.3
bC2H2 = 0.08664 P = 0.08664
= 0.001 389
6140
c
Now the mixture parameters are from Eq.12.84 so we need the mass fractions
y M 0.6 × 28.054
cC2H4 = M
=
= 0.6175,
cC2H4 = 1 - cC2H4 = 0.3825
27.26
mix
AE
A
E
A
A
E
E
A
A
E
A
A
E
A
A
E
amix =
A
2
∑ ci a1/2
i = (0.6175 × 9.9863 + 0.3825 × 11.8462) = 10.679
2
E
E AE
EA
A
A
EA
A
A
bmix = ∑ ci bi = 0.6175 × 0.001 439 + 0.3825 × 0.001 389 = 0.001 42
RT
a
Using now Eq.12.53:
P=
−
v − b v(v + b)T1/2
0.305 × 300
10.679
6000 =
−
v − 0.001 42 v(v + 0.001 42) 3001/2
By trial and error we find the specific volume, v = 0.006683 m3/kg
Kay’s rule Eq.12.84
Pc mix = 0.6 × 5.04 + 0.4 × 6.14 = 5.48 MPa
Tc mix = 0.6 × 282.4 + 0.4 × 308.3 = 292.8 K
6
300
Reduced properties: Pr = 5.48 = 1.095, Tr = 292.8 = 1.025
Fig. D.1: Z = 0.4 (difficult to read)
v = ZRT/P = 0.4 × 0.305 × 300 / 6000 = 0.0061 m3/kg
A
A
A
A
E
A
A
E
A
E
E
E
A
A
A
E
A
A
A
E
E
A
A
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.128
For the previous problem, find the specific heat transfer using Kay’s rule and the
generalized charts.
To do the EOS we need the gas constant, so from Eq.11.5 we get
Mmix = ∑ yi Mi = 0.6 × 28.054 + 0.4 × 26.068 = 27.26
Rmix = 8.3145/27.26 = 0.305 kJ/kg K
y M 0.6 × 28.054
cC2H4 = M
=
= 0.6175,
cC2H4 = 1 - cC2H4 = 0.3825
27.26
mix
A
A
E
CP mix = ∑ ci CP i = 0.6175 × 1.548 + 0.3825 × 1.699 = 1.606 kJ/kg K
Kay’s rule Eq.12.84
Pc mix = 0.6 × 5.04 + 0.4 × 6.14 = 5.48 MPa
Tc mix = 0.6 × 282.4 + 0.4 × 308.3 = 292.8 K
6
300
Reduced properties 1:
Pr1 = 5.48 = 1.095, Tr1 = 292.8 = 1.025
A
A
A
A
E
*
h1 − h1
(
Fig. D.1:
A
A
) = 2.1 × RTc = 2.1 × 0.305 × 292.8 = 187.5 kJ/kg
A
E
E
A
A
A
E
E
6
Pr2 =
= 1.095,
5.48
Reduced properties 2:
A
Tr2 =
A
E
*
h2 − h2
(
Fig. D.1:
A
A
A
E
) = 0.7 × RTc = 0.7 × 0.305 × 292.8 = 62.5 kJ/kg
A
E
400
= 1.366
292.8
A
A
A
A
E
E
The energy equation gives
1q2 = (h2 - h1) =
A
A
E
A
A
E
A
A
E
A
E
A
(h2 − h*2) + (h*2 − h*1) + (h*1 − h1)
A
A
E
A
A
E
A
A
A
E
A
A
E
A
E
A
A
E
= -62.5 + 1.606 (400 – 300) + 187.5
= 285.6 kJ/kg mix
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.129
A gas mixture of a known composition is frequently required for different
purposes, e.g., in the calibration of gas analyzers. It is desired to prepare a gas
mixture of 80% ethylene and 20% carbon dioxide (mole basis) at 10 MPa, 25°C
in an uninsulated, rigid 50-L tank. The tank is initially to contain CO2 at 25°C
and some pressure P1. The valve to a line flowing C2H4 at 25°C, 10 MPa, is now
opened slightly, and remains open until the tank reaches 10 MPa, at which point
the temperature can be assumed to be 25°C. Assume that the gas mixture so
prepared can be represented by Kay’s rule and the generalized charts. Given the
desired final state, what is the initial pressure of the carbon dioxide, P1?
A = C2H4, B = CO2
A
A
A
A
E
Pi =10 MPaA
o
Ti = 25 C
A
E
E
T1 = 25 oC
E
A
A
A
A
E
P2 = 10 MPa, T2 = 25 oC
E
A
A
A
A
E
A
A
V=0.05 3
m B
E
yA2 = 0.8, yB2 = 0.2
A
A
A
A
E
E
Mixture at 2 :
PC2 = 0.8 × 5.04 + 0.2 × 7.38 = 5.508 MPa
A
A
E
TC2 = 0.8 × 282.4 + 0.2 × 304.1 = 286.7 K
A
A
E
Tr2 = 298.15/286.7 = 1.040; Pr2 = 10/5.508 = 1.816
A
A
A
E
A
E
D.1 :
Z2 = 0.32
A
A
E
P2V
10 000×0.05
- = 0.32×8.3145×298.2 = 0.6302 kmol
Z2RT2
n2 =
A
A
A
A
E
A
A
E
E
E
nA2 = ni = 0.8 n2 = 0.5042 kmol C2H4
A
A
A
E
A
A
A
A
E
A
A
E
E
nB2 = n1 = 0.2 n2 = 0.1260 kmol CO2
A
A
A
A
E
A
A
E
E
A
E
E
298.2
Tr1 = 304.1 = 0.981
A
A
A
E
A
E
n1ZB1RT1
0.126 ZB1× 8.3145×298.2
E
Pr1 =
A
A
E
A
PCBV
A
=
7380×0.05
A
E
E
E
A
= 0.8466 ZB1
A
E
By trial & error: Pr1 = 0.618 & ZB1 = 0.73
A
A
A
E
A
E
⇒ P1 = 0.618 × 7.38 = 4.56 MPa
A
A
E
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.130
One kmol/s of saturated liquid methane, CH4, at 1 MPa and 2 kmol/s of ethane,
C2H6, at 250°C, 1 MPa are fed to a mixing chamber with the resultant mixture
exiting at 50°C, 1 MPa. Assume that Kay’s rule applies to the mixture and
determine the heat transfer in the process.
Control volume the mixing chamber, inlet CH4 is 1, inlet C2H6 is 2 and the
exit state is 3. Energy equation is
.
. . . QCV = n3 h3 - n1 h1 - n2 h2
E
E
E
E
E
A
A
A
A
A
E
A
E
A
E
A
A
A
A
A
E
A
A
A
A
E
A
A
E
Select the ideal gas reference temperature to be T3 and use the
generalized charts for all three states.
Pr1 = Prsat = 1/4.60 = 0.2174 =>
Trsat = 0.783,
T1 = 0.783 × 190.4 = 149.1 K,
∆h1 = 4.57
Pr2 = 1/4.88 = 0.205, Tr2 = 523/305.4 = 1.713,
∆h2 = 0.08
h1 = C1(T1 - T3) - ∆h1 RTc = 36.15(149.1 - 323.2) - 4.57 × 8.3145 × 190.4
E
E
A
A
E
A
A
A
A
= -13528 kJ/kmol
h2 = C2(T2 - T3) - ∆h2 RTc = 53.11(250 - 50) - 0.08 × 8.3145 × 305.4
E
E
A
A
A
E
A
A
A
= 10 419 kJ/kmol
Kay’s rule Eq.12.84
Tcmix = (1 × 190.4 + 2 × 305.4)/3 = 267.1 K
Pcmix = (1 × 4.60 + 2 × 4.88)/3 = 4.79 MPa
Tr3 = 323.2/267.1 = 1.21 ,
Pr3 = 1/4.79 = 0.21,
∆h3 = 0.15
h3 = 0 - 0.15 × 267.1 × 8.3145 = - 333 kJ/kmol
E
A
A
.
QCV = 3(-333) - 1(-13528) - 2(10 419) = - 8309 kW
E
A
A
A
E
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.131
Saturated-liquid ethane at T1 = 14°C is throttled into a steady flow mixing
chamber at the rate of 0.25 kmol/s. Argon gas at T2 = 25°C, P2 = 800 kPa, enters
the chamber at the rate of 0.75 kmol/s. Heat is transferred to the chamber from a
heat source at a constant temperature of 150oC at a rate such that a gas mixture
exits the chamber at T3 = 120oC, P3 = 800 kPa. Find the rate of heat transfer and
the rate of entropy generation.
.
Argon, Ta2 = 25oC, P2 = 800 kPa, n2 = 0.75 kmol/s
A
A
E
A
A
A
E
A
E
E
A
A
E
A
A
A
A
A
A
E
E
Tca = 150 K, Pca = 4.87 MPa, Ma = 39.948 kg/kmol, Cpa = 0.52 kJ/kg K
ha3 - ha2 = MaCpa(T3 - Ta2) = 1973.4 kJ/kmol
.
Inlet: Ethane, Tb1 = 14oC, sat. liq., xb1 = 0, n1 = 0.25 kmol/s
Tcb = 305.4 K, Pcb = 4.88 MPa, Mb = 30.07 kg/kmol, Cpb = 1.766 kJ/kg-K
Tr1 = 0.94, Pb1 = Pr1Pcb = 0.69 × 4880 = 3367 kPa
∗
−∗ −
−
−
hb1 − hb1 = 3.81 RTcb = 9674.5 kJ/kmol, s-b1 − s-b1 = 3.74 R = 31.1
E
A
A
−∗ −∗
hb3 - h b1 = MbCpb(T3 - Tb1) = 5629.6 kJ/kmol
Exit: Mix, T3 = 120oC, P3 = 800 kPa consider this an ideal gas mixture.
. . . . . . Energy Eq.: n1hb1 + n2ha2 +Q = n3h3 = n1hb3 + n2ha3
E
A
A
. . . Q = n1(hb3 - hb1) + n2(ha3 - ha2) = 0.25 (5629.6 + 9674.5) + 0.75(1973.4)
E
A
A
= 5306 kW
.
.
.
.
Entropy Eq.: Sgen = n1(s-b3 − s-b1) + n2(s-a3 − s-a2) - Q/TH ;
E
A
A
TH = 150oC
. .
ya = n2/ntot = 0.75;
. .
yb = n1/ntot = 0.25
T3 − yaP3
s-a3 − s-a2 = MaCpalnT - R ln P = 8.14 kJ/kmol-K
a2
a2
E
A
A
T3 − ybP3 ∗
s-b3 − s-b1 = MbCpblnT - R ln P + s-b1 − s-b1 =
E
A
b1
A
b1
= 40.172 + 31.1 = 71.27 kJ/kmol K
.
Sgen = 0.25 × 71.27 + 0.75 × 8.14 - 5306 / 423 = 11.38 kW/K
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.132
A cylinder/piston contains a gas mixture, 50% CO2 and 50% C2H6 (mole basis)
at 700 kPa, 35°C, at which point the cylinder volume is 5 L. The mixture is now
compressed to 5.5 MPa in a reversible isothermal process. Calculate the heat
transfer and work for the process, using the following model for the gas mixture:
a. Ideal gas mixture.
b. Kay’s rule and the generalized charts.
a) Ideal gas mixture
U2 - U1 = mCv mix(T2 - T1) = 0
A
A
E
Q12 = W12 = ⌠ P dV = P1V1 ln(V2/V1) = - P1V1 ln(P2/P1)
⌡
A
EA
= - 700 × 0.005 ln(5500/700) = -7.71 kJ
b) Kay's rule
Tcmix = 0.5 × 304.1 + 0.5 × 305.4 = 304.75 K
Pcmix = 0.5 × 7.38 + 0.5 × 4.88 = 6.13 MPa
Tr1 = 308.15/304.75 = 1.011, Pr1 = 0.7/6.13 = 0.1142
Z1 = 0.96, ∆h1 = 0.12, ∆s1 = 0.08
700*0.005
n = P1V1/Z1R T1 = 0.962*8.3145*308.15 = 0.00142 kmol
Tr2 = Tr1 , Pr2 = 5.5/6.13 = 0.897,
E
A
A
A
A
E
∆h2 = 1.35,
Z2 = 0.58,
∆s2 = 1.0
- - h2 - h1 = (h2 - h1) - R Tc(∆h2 - ∆h1)
E
E
A
A
E
A
A
A
E
A
A
E
A
A
A
= 0 - 8.3145 × 304.75(1.35 - 0.12) = - 3117
- u-2 - u-1 = h2 - h1 + RT(Z1 - Z2) = - 3117
E
E
A
E
E
E
A
A
A
A
A
A
A
A
A
+ 8.3145 × 308.15(0.96 - 0.58) = -2143 kJ/kmol
Q12 = nT(s-2 - s-1)T = 0.00142 × 308.15 × 8.3145[ 0 - ln(5.5/0.7) -1.0
E
A
W12 = Q12
E
A
A
A
+ 0.08 ] = - 10.85 kJ
- n(u- - u- ) = -10.85 - 0.00142(-2143) = - 7.81 kJ
E
A
2
A
E
A
1
A
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.133
A cylinder/piston contains a gas mixture, 50% CO2 and 50% C2H6 (mole basis) at
700 kPa, 35°C, at which point the cylinder volume is 5 L. The mixture is now
compressed to 5.5 MPa in a reversible isothermal process. Calculate the heat
transfer and work for the process, using the following model for the gas mixture:
a. Ideal gas mixture. b. The van der Waals equation of state.
a) Ideal gas mixture
U2 - U1 = mCv mix(T2 - T1) = 0
A
A
E
Q12 = W12 = ⌠ P dV = P1V1 ln(V2/V1) = - P1V1 ln(P2/P1)
⌡
A
EA
= - 700 × 0.005 ln(5500/700) = -7.71 kJ
b) van der waal's equation
For CO2 : b = R Tc/8Pc = 8.3145 × 304.1/8 × 7380 = 0.04282
E
A
A
a = 27 Pc b2 = 27 × 7380 × 0.042822 = 365.45
b = R Tc/8Pc = 8.3145 × 305.4/8 × 4880 = 0.06504
For C2H6 :
E
A
A
a = 27 Pc b2 = 27 × 4880 × 0.065042 = 557.41
amix = (0.5 365.45 + 0.5 557.41)2 = 456.384
A
EA
A
EA
bmix = 0.5 × 0.04282 + 0.5 × 0.06504 = 0.05393
8.3145*308.2 456.384
- - 700 = 0
v- - 0.05393
v 2
A
A
1
A
A
1
E
E
By trial and error: v-1 = 3.5329 m3/kmol
E
E
A
A
A
A
8.3145*308.2 456.384
- 5500 = 0
v- - 0.05393
v- 2
A
A
2
A
A
2
E
E
By trial and error: v-2 = 0.2815 m3/kmol
E
A
E
A
A
A
n = V1/v-1 = 0.005/3.5329 = 0.00142
v-2 - b
Q12 = nT(s-2 - s-1)T = n R T ln
v-1 - b
E
A
A
E
E
A
E
A
A
A
A
A
A
E
E
0.2815 - 0.05392
= 0.00142 × 8.3145 × 308.2 ln 3.5329 - 0.05392 = - 9.93 kJ
A
A
E
U2 - U1 = 0.00142 × 456.39(3.5329-1 - 0.2815-1) = -2.12 kJ
Q12 = U2 - U1 + W12 => W12 = -9.93 -(-2.12) = -7.81 kJ
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
Helmholtz EOS
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.134
Verify that the ideal gas part of Helmholtz function substituted in Eq.12.89
does lead to the ideal gas law as in note after Eq.12.97.
The ideal gas Helmholtz function is from its definition, see Eq.12.12,
a* = u* – Ts* = h* – RT – T s*
We have
E
A
E
A
A
E
A
A
E
A
A
E
A
A
*
h* = ho + ⌡
⌠ CPo dT
E
A
A
A
A
E
* ⌠ CPo
ρT
s* = so + T dT – R ln
⌡
ρoTo
∂a*
Now from Eq.12.89 we need to look at so following 12.92
∂ρ T
*
∂h
∂a*
∂s*
∂RT
= –
–T
∂ρ T ∂ρ T ∂ρ T
∂ρ T
ρT
∂
= 0 – 0 + TR ln
∂ρ ρoToT
= RT / ρ
So then
∂a*
ρ2 = P = ρRT
Ideal gas OK
∂ρ T
E
A
A
A
A
E
A
A
E
A
A
E
A
A
E
E
A
A
E
E
A
E
A
E
E
A
E
E
A
A
A
E
E
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.135
Gases like argon and neon have constant specific heats. Develop an expression
for the ideal gas contribution to Helmholtz function in Eq.12.92 for these cases.
The ideal gas Helmholtz function is from its definition, see Eq.12.92,
a* = u* – Ts* = h* – RT – T s*
We have
E
A
E
A
A
E
A
A
E
A
A
E
A
A
*
*
h* = ho + ⌡
⌠ CPo dT = ho + CPo(T – To)
E
A
A
A
A
A
A
E
E
* ⌠ CPo
ρT
s* = so + T dT – R ln
⌡
ρoTo
*
ρT
= so + CPo ln(T /To) – R ln
ρoTo
So now we get
E
A
A
A
A
E
A
A
E
*
a* = ho + CPo(T – To) – RT – T s*
E
A
E
A
A
A
A
E
T
*
*
ρT
= ho – T so + CPo(T – To) – CPoT ln ( T ) – RT + RT ln
o
ρoTo
T
ρ
= Co + C1 T – C2 T ln ( T ) + RT ln
o
ρo
A
A
A
A
E
E
where
*
Co = ho – CPoTo ;
A
A
E
*
C1 = CPo – R – so ;
A
E
A
C2 = CPo – R
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.136
Find an expression for the change in Helmholtz function for a gas with an EOS as
P(v – b) = RT.
From Eq.12.31 we get
duT = [ T (
A
∂P
R
)
– P ] dvT = [ T ( v – b ) – P ] dvT = [ P – P] dvT = 0
v
∂T
A
A
A
A
A
E
E
E
From Eq.12.32 or Eq.12.34 we get
∂v
∂P
dsT = – ( )P dPT = ( )v dvT
∂T
∂T
A
A
A
A
A
A
E
A
A
E
E
E
R
R
= – P dPT = v – b dvT
A
A
A
A
E
E
Now the changes in u and s can be integrated to find
(u2 – u1)T = 0
P2
v2 – b
(s2 – s1)T = –R ln P = R ln – b
v1
1
to this we now need to add the variation due to T. For this we get
2
2
(u2 – u1)v = ⌡
⌠ Cv dT
and
1
⌠ Cv
(s2 – s1)v = T dT
⌡
1
Finally since the Helmholtz function contains the product Ts we need the absolute
value of the entropy so
1
v1 – b
⌠ Cv
s1 = so + T dT + R ln – b
vo
⌡
o
Then the change in Helmholtz function becomes
a2 – a1 = u2 – u1 – T2s2 + T1s1 = u2 – u1 – T2(s2 – s1) + (T1 –T2) s1
2
2
v2 – b
⌠ Cv
=⌠
C
dT
–
T
[
dT
+
R
ln
v
2
⌡
v1 – b ] + (T1 – T2) s1
⌡ T
1
1
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.137
Use the equation of state in Example 12.3 and find an expression for isothermal
changes in Helmholtz function between two states.
The EOS is
RT C
v= P – 3
T
and we use the isothermal changes found in Ex.14.3 as
4C
(h2 – h1)T = – 3 (P2 – P1)T
T
P2
3C
(s2 – s1)T = – R ln P – 4 (P2 – P1)T
1T T
A
Pv
P
=
1
–
C’
RT
T4
A
A
or
A
A
E
A
A
E
E
A
E
A
E
A
A
E
As Helmholtz function is: a = u – Ts; we get
(a2 – a1)T = (u2 – u1)T – T(s2 – s1)T
= (h2 – h1)T – (P2v2 – P1v1) – T(s2 – s1)T
4C
C
= – 3 (P2 – P1)T – [RT2 – RT1 – 3(P2 – P1)T ]
T
T
P2
3C
+ RT ln P + 3 (P2 – P1)T
1T T
A
A
A
E
A
E
A
A
E
P2
4C
C
3C
= – 3 (P2 – P1)T + 3(P2 – P1)T + RT ln P + 3 (P2 – P1)T
T
T
1T T
This now reduces to the final answer
P2
(a2 – a1)T = RT ln P
1T
A
A
E
A
A
E
A
A
E
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.138
Assume a Helmholtz equation as
T
ρ
a* = Co + C1 T – C2 T ln ( T ) + RT ln ( ρ )
E
A
A
o
o
where Co, C1, C2 are constants and To and ρo reference values for temperature
and density, see Eqs. 12.92 - 95. Find the properties P, u and s from this
expression. Is anything assumed for this particular form.
Given Helmholtz function we can find the pressure and entropy from Eq. 12.21
and then u from the definition: a = u – Ts.
T
v
a* = Co + C1 T – C2 T ln ( T ) – RT ln ( v )
o
o
E
A
A
v
∂
∂a*
= – RT ln ( v ) = – RT / v
∂v
∂v T
o
*
∂a
P = – = RT / v
i.e. Ideal gas.
∂v T
A
A
A
E
E
A
A
E
A
E
E
T
v
∂a*
s = – = – C1 + C2 ln ( T ) + C2 + R ln ( v )
∂T v
o
o
Notice how it looks like Eq.6.17.
T
v
u = a + Ts = Co + C1 T – C2 T ln ( T ) – RT ln ( v )
o
o
T
v
– C1T + C2T ln ( T ) + C2T + RT ln ( v )
o
o
= Co + C2 T
We find that u is linear in T.
A
A
E
E
Not only is it ideal gas but it also has constant specific heats.
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
Review Problems
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.139
Saturated liquid ethane at 2.44 MPa enters a heat exchanger and is brought to 611
K at constant pressure, after which it enters a reversible adiabatic turbine where it
expands to 100 kPa. Find the heat transfer in the heat exchanger, the turbine exit
temperature and turbine work.
From D.2,
Pr1 = 2.44/4.88 = 0.50 , Tr1 = 0.89, T1 = 0.89×305.4 = 271.8 K
A
A
A
A
E
A
E
A
E
*
(h1-h1) = 0.2765×305.4×4.12 = 347.9
A
A
A
A
E
E
* *
(h2-h1) = 1.766 (611 - 271.8) = 599.0
A
A
A
A
E
E
Pr2 = 0.50 , Tr2 = 611/305.4 = 2.00
A
A
A
A
E
E
*
(h2-h2) = RTc × 0.14 = 0.2765×305.4×0.14 = 11.8
From D.2:
A
A
A
A
A
E
A
E
E
q = (h2-h1) = -11.8 + 599.0 + 347.9 = 935.1 kJ/kg
A
A
A
A
E
E
From D.3,
*
(s2-s2) = 0.2765×0.05 = 0.0138
A
A
A
A
E
E
T3
100
* *
(s3-s2) = 1.766 ln 611 - 0.2765 ln 2440
A
A
A
A
A
E
A
A
E
E
E
E
Assume T3 = 368 K , Tr3 = 1.205
A
A
A
E
A
E
at Pr3 = 0.020
A
A
E
* *
(s3-s2) = -0.8954 + 0.8833 = -0.0121
A
A
A
A
E
E
From D.3,
*
(s3-s3) = 0.2765×0.01 = 0.0028
A
A
A
A
E
E
(s3-s2) = -0.0028 - 0.0121 + 0.0138 ≈ 0
A
A
A
A
E
ΟΚ
E
Therefore, T3 = 368 K
A
A
E
From D.2,
*
(h3-h3) = 0.2765×305.4×0.01 = 0.8
A
A
A
A
E
E
w = (h2-h3) = -11.8 + 1.766 (611 - 368) + 0.8 = 418.1 kJ/kg
A
A
A
E
A
E
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.140
A piston/cylinder initially contains propane at T = -7°C, quality 50%, and volume
10L. A valve connecting the cylinder to a line flowing nitrogen gas at T = 20°C, P
= 1 MPa is opened and nitrogen flows in. When the valve is closed, the cylinder
contains a gas mixture of 50% nitrogen, 50% propane on a mole basis at T =
20°C, P = 500 kPa. What is the cylinder volume at the final state, and how much
heat transfer took place?
State 1: Propane, T = -7oC, x = 0.5, V = 10 L
A
1
1
A
A
E
1
A
A
E
A
E
Tc = 369.8 K, Pc = 4.25 kPa, CP = 1.679 kJ/kg-K, M = 44.097 kg/kmol
Tr1 = 0.72, Pr1 = 0.12, P1 = Pr1Pc = 510 kPa
Zf1 = 0.020, Zg1 = 0.88, Z1 = (1 - x1)Zf1 + x1Zg1 = 0.45
Fig. D.1:
A
A
E
Fig. D.1:
A
A
E
−
n1 = P1V1/(Z1RT1) = 510 × 0.01/(0.45 × 8.3145 × 266.2) = 0.00512 kmol
E
A
A
A
A
E
A
A
A
A
E
E
A
A
A
E
E
− −* −
− −*
−*
h1 = h 1o + CP(T1 - To) + (h 1 - h 1 ) ; h1o = 0,
A
A
A
A
E
E
−* −
−
(h 1-h 1)f /RTc = 4.79,
A
A
E
E
A
−* −
−
(h1-h 1)g /RTc = 0.25
A
E
E
−* −
−* −
−* −
h 1 - h 1 = (1 - x1) (h 1 - h 1)f + x1 (h 1 - h 1)g = 7748 kJ/kmol
A
A
A
E
E
A
A
E
A
E
E
E
−
h 1 = 0 + 1.679 × 44.094(-7 - 20) - 7748 = -9747 kJ/kmol
Inlet: Nitrogen, Ti = 20oC, Pi = 1.0 MPa,
Tc = 126.2 K, Pc = 3.39 MPa, Cpn = 1.042 kJ/kg-K, M = 28.013 kg/kmol
−* −
Tri = 2.323, Pri = 0.295, h i -h i = 0.06 × 8.3145 × 126.2 = 62.96 kJ/kmol
− −*
−*
− −* −
h i = hio + CPn(Ti - To) + (hi - h i ) ; hio = 0, Ti - To = 0
A
A
E
A
E
A
E
State 2: 50% Propane, 50% Nitrogen by mol, T2 = 20oC, P2 = 500 kPa
Tcmix = ∑yiTci = 248 K,
A
A
A
E
A
E
A
A
E
Pcmix = ∑yiPci = 3.82 MPa
A
A
E
A
A
A
A
E
E
−* − −
Tr2 = 1.182, Pr2 = 0.131, Z2 = 0.97, (h 2 - h2)/RTc = 0.06
− −*
−*
− −* −
h2 = h 2o + CPmix(T2 - To) + (h 2 - h 2 ) ; h2o = 0, T2 - To = 0
A
A
E
A
E
A
E
a) ni = n1 => n2 = n1 + ni = 0.1024,
−
V2 = n2Z2RT2/P2 = 0.0484 m3
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
b) Energy Eq.:
Qcv + nihi = n2u-2 - n21u-21 + Wcv;
u- = h - PvE
A
E
A
A
E
Wcv = (P1 + P2)(V2 - V1)/2 = 19.88 kJ
Qcv = n2h2 - n1h1 - nihi - P2V2 + P1V1 + Wcv
−
−
h i = -62.96 kJ/kmol, h2 = -123.7 kJ/kmol, Qcv = 50.03 kJ
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.141
A newly developed compound is being considered for use as the working fluid in
a small Rankine-cycle power plant driven by a supply of waste heat. Assume the
cycle is ideal, with saturated vapor at 200°C entering the turbine and saturated
liquid at 20°C exiting the condenser. The only properties known for this
compound are molecular weight of 80 kg/kmol, ideal gas heat capacity CPO= 0.80
A
A
E
kJ/kg K and TC = 500 K, PC = 5 MPa. Calculate the work input, per kilogram, to
A
A
A
E
A
E
the pump and the cycle thermal efficiency.
T1 = 200oC = 473.2 K, x1 = 1.0
1
E
A
A
A
A
A
A
E
.
WT
Turbine
.
QH
E
o
T3 = 20 C = 293.2 K, x3 = 0.0
A
A
A
E
A
A
E
Properties known:
M = 80, CPO = 0.8 kJ/kg K
A
A
E
Ht.
Exch
TC = 500 K, PC = 5.0 MPa
473.2
293.2
Tr1 = 500 = 0.946 , Tr3 = 500 = 0.586
2
A
A
A
E
Cond
A
A
P
4
.
-WP
A
A
A
A
A
E
E
A
E
R = R/M = 8.31451/80 = 0.10393 kJ/kg K
3
From Fig. D.1,
Pr1 = 0.72, P1 = 0.72 × 5 = 3.6 MPa = P4
A
A
E
E
A
A
E
A
A
A
A
E
E
E
Pr3 = 0.023, P3 = 0.115 MPa = P2 , ZF3 = 0.004
A
A
A
A
E
A
E
vF3 =
A
A
E
A
A
E
A
E
ZF3RT3 0.004 × 0.10393 × 293.2
= 0.00106 m3/kg
P3 =
115
E
A
A
E
A
A
E
A
A
E
4
wP = - ⌠
⌡ vdP ≈ vF3(P4 -P3) = -0.00106(3600-115) = -3.7 kJ/kg
A
A
A
EA
A
A
E
A
E
A
A
A
E
E
3
qH + h4 = h1 , but h3 = h4 + wP
A
A
A
E
A
A
E
A
A
E
A
E
A
=>
A
E
A
A
E
qH = (h1-h3) + wP
A
A
A
E
A
A
E
A
E
A
A
E
From Fig. D.2:
*
(h1-h1) = RTC × 1.25 = 0.103 93 × 500 × 1.25 = 64.9 kJ/kg
A
A
A
A
A
A
E
E
E
*
(h3-h3) = 0.103 93 × 500 × 5.2 = 270.2 kJ/kg
A
A
A
A
E
E
* *
(h1-h3) = CP0(T1-T3) = 0.80(200-20) = 144.0 kJ/kg
A
A
A
A
E
A
A
A
A
A
A
E
E
E
E
(h1-h3) = -64.9 + 144.0 + 270.2 = 349.3 kJ/kg
A
A
A
E
A
E
qH = 349.3 + (-3.7) = 345.6 kJ/kg
A
A
E
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
*
*
*
*
Turbine, (s2 - s1) = 0 = -(s2 - s2)+(s2 - s1) + (s1 - s1)
A
A
A
A
A
A
A
A
A
E
E
A
A
A
E
E
A
E
A
A
A
E
E
E
From Fig. D.3,
*
(s1-s1) = 0.10393×0.99 = 0.1029 kJ/kg K
A
A
A
A
E
E
293.2
115
* *
(s2-s1) = 0.80 ln 473.2 - 0.103 93 ln 3600 = -0.0250
A
A
A
A
E
A
A
A
A
E
E
E
Substituting,
*
*
s2-s2 = +0.1029 - 0.0250 = 0.0779 = (s2-sF2) - x2sFG2
A
A
A
A
A
E
A
A
A
A
A
A
E
E
E
E
0.0779 = 0.103 93×8.85 - x2×0.103 93(8.85-0.06)
A
A
E
*
*
(h2-h2) = (h2-hF2) - x2hFG2
A
A
A
A
A
A
E
A
A
A
E
=> x2 = 0.922
A
A
E
A
A
E
E
E
E
E
From Fig. D.2,
hFG2 = 0.10393 × 500 (5.2-0.07) = 266.6
A
A
E
*
(h2-h2) = 270.2 -0.922 × 266.6 = 25.0
A
A
A
A
E
E
wT = (h1-h2) = -64.9 + 144.0 + 25.0 = 104.1 kJ/kg
A
A
A
E
A
A
E
A
E
ηTH =
A
A
E
wNET
qH
A
E
E
A
=
104.1-3.7
345.6 = 0.29
A
A
E
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.142
A 200-L rigid tank contains propane at 400 K, 3.5 MPa. A valve is opened, and
propane flows out until half the initial mass has escaped, at which point the valve
is closed. During this process the mass remaining inside the tank expands
according to the relation Pv1.4 = constant. Calculate the heat transfer to the tank
during the process.
C3H8: V = 200 L, T1 = 400 K, P1 = 3.5 MPa
E
A
A
A
A
A
A
E
A
A
A
E
A
E
E
1.4
Flow out to m2 = m1/2 ; Pv
A
A
A
A
E
= const inside
E
A
A
E
400
3.5
Tr1 = 369.8 = 1.082, Pr1 = 4.25 = 0.824 Fig D.1: Z1 = 0.74
A
A
A
A
A
A
E
A
A
A
E
A
E
E
E
0.74×0.188 55×400
= 0.01594, v2 = 2v1 = 0.03188
3500
0.2
1
m1 = 0.015 94 = 12.55 kg, m2 = 2 m1 = 6.275 kg,
v1 =
A
A
A
A
A
A
E
A
A
E
E
E
A
A
A
A
A
A
E
A
A
A
E
A
E
E
E
v1 1.4 3500
P2 = P1 v
= 1.4 = 1326 kPa
2
2
Trial & error: saturated with
1.326
T = 0.826×369.8 = 305.5 K &
Pr2 = 4.25 = 0.312
2
Z = 1326×0.03188 = 0.734
P2v2 = Z2RT2
2 0.188 55×305.5
Z2 = ZF2 + x2(ZG2 - ZF2) = 0.734 = 0.05 + x2(0.78-0.05) => x2 = 0.937
A
( )
A
A
A
E
A
A
E
A
A
A
A
E
E
E
E
E
A
E
A
A
E
A
A
A
A
E
A
A
E
A
A
E
E
A
A
E
A
E
A
A
E
A
E
*
(h1-h1) = 0.188 55×369.8(0.9) = 62.8
* *
(h2-h1) = 1.6794(305.5-400) = -158.7
A
A
A
A
E
E
A
A
A
A
E
E
*
[
*
]
(h2-h2) = (h2-hF2) - x2hFG2 = 0.188 55×369.8 4.41 - 0.937(4.41-0.55)
A
A
A
A
E
A
A
A
A
E
E
= 55.3
Energy Eq.:
A
A
E
A
A
A
E
*
*
A
A
A
E
A
E
A
A
E
A
A
E
A
A
E
A
A
E
A
A
E
E
*
h1 = 0 + (h1-h1) = -62.8
A
A
E
*
A
E
QCV = m2h2 - m1h1 + (P1-P2)V + mehe AVE
Let h1 = 0 then
A
A
E
A
A
E
A
A
E
*
A
E
*
h2 = h1 + (h2-h1) + (h2-h2) = 0 - 158.7 – 55.3 = -214.0
A
A
A
A
A
A
E
A
A
E
A
A
A
E
E
A
E
E
he AVE = (h1+h2)/2 = -138.4
A
A
A
E
A
E
A
A
E
QCV = 6.275(-214.0) - 12.55(-62.8)
A
A
E
+ (3500-1326)×0.2 + 6.275(-138.4) = -981.4 kJ
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.143
One kilogram per second water enters a solar collector at 40°C and exits at 190°C,
as shown in Fig. P12.143. The hot water is sprayed into a direct-contact heat
exchanger (no mixing of the two fluids) used to boil the liquid butane. Pure
saturated-vapor butane exits at the top at 80°C and is fed to the turbine. If the
butane condenser temperature is 30°C and the turbine and pump isentropic
efficiencies are each 80%, determine the net power output of the cycle.
H2O cycle: solar energy input raises 1 kg/s of liquid H2O from 40oC to 190oC.
E
A
A
A
A
A
E
E
A
A
A
E
Therefore, corresponding heat input to the butane in the heat exchanger is
.
.
QH = m(hF 190 C-hF 40 C)H2O = 1(807.62-167.57) = 640.05 kW
E
E
A
A
A
A
A
A
A
A
E
A
A
E
A
E
E
C4H10 cycle
1
A
A
A
A
E
Turbine
E
T1 = 80 oC, x1 = 1.0 ; T3 = 30 oC, x3 = 0.0
.
WT
E
A
A
A
A
A
A
A
E
A
A
E
ηST = ηSP = 0.80
353.2
Tr1 = 425.2 = 0.831
From D.1, D.2 and D.3:
P1 = 0.325×3800 = 1235 kPa
A
A
Ht.
Exch
A
E
A
A
E
.
QH
E
A
A
E
A
2
E
A
A
A
E
Cond
E
A
A
E
*
(h1-h1) = 0.143 04×425.2×0.56 = 34.1
*
(s1-s1) = 0.143 04×0.475 = 0.0680
P
A
A
A
A
E
E
4
.
-WP
3
A
A
A
A
E
E
303.2
Tr3 = 425.2 = 0.713
A
A
A
A
E
E
From D.1, D.2 and D.3: P3 = 0.113×3800 = 429 kPa
A
A
E
sat. liq.: (h*-hF) = RTC×4.81 = 292.5 ;
(s*-sF) = R×6.64 = 0.950
E
A
A
A
A
A
E
A
A
E
E
sat. vap.: (h -hG) = RTC×0.235 = 14.3 ;
*
(s -sG) = R×0.22 = 0.031
E
A
A
A
E
A
A
E
A
E
*
A
A
A
A
E
A
A
A
E
Because of the combination of properties of C4H10 (particularly the large CP0
A
A
A
E
A
A
E
/R), s1 is larger than sG at T3. To demonstrate,
A
A
A
A
E
A
A
E
A
E
353.2
1235
* *
(s1-sG3) = 1.7164 ln 303.2 - 0.143 04 ln 429 = 0.1107
A
A
A
A
E
A
A
A
A
E
E
E
(s1-sG3) = -0.0680 + 0.1107 + 0.031 = +0.0737 kJ/kg K
A
A
E
A
A
E
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
E
Borgnakke and Sonntag
T
so that T2S will be > T3, as shown in the T-s
diagram. A number of other heavy hydrocarbons
also exhibit this behavior.
Assume T2S = 315 K, Tr2S = 0.741
A
1
A
E
2s 2
3
A
A
A
E
A
A
A
E
E
s
From D.2 and D.3:
*
(h2S-h2S) = RTC×0.21 = 12.8
A
A
A
A
A
E
*
and
A
E
(s2S-s2S) = R×0.19 = 0.027
A
A
A
A
E
E
E
353.2
1235
* *
(s1-s2S) = 1.7164 ln 315 - 0.143 04 ln 429 = +0.0453
A
A
A
A
A
E
A
A
A
E
E
E
(s1-s2S) = -0.0680 + 0.0453 + 0.027 ≈ 0
A
A
A
A
E
E
⇒ T2S = 315 K
A
A
E
*
*
(h1-h2S) = 1.7164(353.2-315) = 65.6
A
A
A
A
E
E
wST = h1-h2S = -34.1 + 65.6 + 12.8= 44.3 kJ/kg
A
A
A
A
E
A
A
E
E
wT = ηS×wST = 0.80×44.3 = 35.4 kJ/kg
A
A
A
A
E
A
A
E
E
At state 3,
0.019×0.143 04×303.2
v3 =
= 0.001 92 m3/kg
429
E
A
A
A
A
E
A
A
E
-wSP ≈ v3(P4-P3) = 0.001 92(1235-429) = 1.55 kJ/kg
A
A
A
A
E
-wP =
A
A
ηSP
A
A
A
E
-wSP
E
A
A
E
E
E
1.55
= 0.8 = 1.94 kJ/kg
A
A
A
E
E
wNET = wT + wP = 35.4 - 1.94= 33.46 kJ/kg
A
A
A
A
A
E
A
E
E
For the heat exchanger,
.
.
QH = 640.05 = mC4H10(h1-h4)
E
E
A
A
A
A
A
A
E
A
But
E
h1-h4 = h1-h3+wP
A
A
A
A
E
A
A
E
A
E
A
A
A
A
A
E
E
A
E
E
*
* *
*
h1-h3 = (h1-h1) + (h1-h3) + (h3-h3)
A
A
A
A
A
A
A
A
A
E
E
E
A
E
A
A
A
E
A
A
A
E
E
E
= -34.1 + 1.716(80 - 30) + 292.5 = 344.2 kJ/kg
Therefore,
640.05
.
mC4H10 = 344.2-1.94 = 1.87 kg/s
.
.
WNET = mC4H10wNET = 1.87 × 33.46 = 62.57 kW
E
A
A
A
A
A
E
E
E
E
A
A
A
E
A
A
A
E
A
A
E
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.144
A piston/cylinder contains ethane gas, initially at 500 kPa, 100 L, and at ambient
temperature, 0°C. The piston is now moved, compressing the ethane until it is at
20°C, with a quality of 50%. The work required is 25% more than would have
been required for a reversible polytropic process between the same initial and
final states. Calculate the heat transfer and the entropy generation for the process.
Ethane: Tc = 305.4 K, Pc = 4.88 MPa,
R = 0.2765 kJ/kg-K, Cp = 1.766 kJ/kg K
Tr1 = 0.895, Pr1 = 0.102 Z1 = 0.95
v1 = Z1RT1/P1 = 0.1435 m3/kg, m1 = V1/v1 = 0.697 kg
State 1:
(h*1 − h1) = 0.13RTc = 11.0 kJ/kg, (s*1 − s1) = 0.09 R = 0.025 kJ/kg K
State 2: T2 = 20oC, x2 = 0.5,
1W2 = 1.25Wrev
Tr2 = 0.96, Pr2 = 0.78, P2 = Pr2Pc = 3806 kPa
Zf2 = 0.14, Zg2 = 0.54, Z2 = (1 - x2)Zf + x2Zg = 0.34
(h*2 − h2) = (1 - x2) 3.65 RTc + x2 (1.39 RTc) = 212.8 kJ/kg
(s*2 − s2) = (1 - x2) 3.45 R + x2 × 1.10 R = 0.629 kJ/kg K
v2 = Z2RT2/P2 = 0.0072 m3/kg,
V2 = mv2 = 0.005 m3
Polytropic process
n
n
P1V1 = P2V2 ,
P2
V1
ln P = n ln V
1
2
n = 0.6783
P2V2 - P1V1
= -96.3 kJ,
1-n
1W2 = 1.25Wrev = -120.4 kJ
Wrev = ∫ P dV =
a) Energy Eq.:
u = h - Pv
1Q2 = m(u2 - u1) + 1W2;
*
*
*
*
h 2 − h2
h2 − h1 + h1 − h1
h2 - h1 = (
)+(
) (
)
= -212.8 + 1.766(20 – 0) + 11.0 = -166.5 kJ/kg
u2 - u1 = (h2 - h1) - (P2v2 - P1v1) = -122.2 kJ/kg
1Q2 = 0.697 kg (-122.2) kJ/kg - 120.4 kJ = -205.6 kJ
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
b) Entropy Eq.:
Sgen = m(s2 - s1) - 1Q2 /To;
*
*
*
To = 0oC = 273.15 K
*
s2 - s1 = (s2 − s2) + (s2 − s1) + (s1 − s1)
(s*2 − s*1) = Cp ln(T2 / T1) − R ln(P2 / P1) = -0.436 kJ/kg K,
205.6
Sgen = 0.697(-0.629 - 0.436 + 0.025) + 273.15 = 0.028 kJ/K
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.145
Carbon dioxide gas enters a turbine at 5 MPa, 100°C, and exits at 1 MPa. If the
isentropic efficiency of the turbine is 75%, determine the exit temperature and the
second-law efficiency using the generalized charts.
CO2 turbine: ηS = w/wS = 0.75
inlet: T1 = 100oC, P1 = 5 MPa, exhaust: P2 = 1 MPa
5
373.2
1
a) Pr1 = 7.38 = 0.678, Tr1 = 304.1 = 1.227, Pr2 = 7.38 = 0.136
From D.2 and D.3,
*
(h1 - h1) = 0.188 92×304.1×0.52 = 29.9
*
(s1 - s1) = 0.188 92×0.30 = 0.0567
Assume T2S = 253 K, Tr2S = 0.832
From D.2 and D.3:
*
(h2S-h2S) = RTC×0.20 = 11.5
*
(s2S - s2S) = R×0.17 = 0.0321
253
1
*
*
(s2S - s1) = 0.8418 ln 373.2 - 0.188 92 ln 5 = -0.0232
(s2S - s1) = -0.0321 - 0.0232 + 0.0567 ≈ 0
⇒ T2S = 253 K
*
*
(h2S - h1) = 0.8418(253-373.2) = -101.2
wS = (h1 - h2S) = -29.9 + 101.2 + 11.5 = 82.8 kJ/kg
*
*
*
*
w = ηS×wS = 0.75×82.8 = 62.1 kJ/kg = (h1-h1) + (h1-h2) + (h2-h2)
Assume T2 = 275 K, Tr2 = 0.904
*
*
(h1 - h2) = 0.8418 (373.2 - 275) = 82.7
From D.2 and D.3,
*
(h2 - h2) = RTC×0.17 = 9.8 ;
*
(s2 - s2) = R×0.13 = 0.0245
Substituting,
w = -29.9 + 82.7 + 9.8 = 62.7 ≈ 62.1
⇒ T2 = 275 K
For the exergies we need the entropy changes
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
275
1
*
*
(s2 - s1) = 0.8418 ln 373.2 - 0.188 92 ln 5 = +0.0470
(s2 - s1) = -0.0245 + 0.0470 + 0.0567 = +0.0792
Assuming T0 = 25 oC,
(ϕ1 - ϕ2) = (h1 - h2) - T0(s1 - s2) = 62.1 + 298.2(0.0792) = 85.7 kJ/kg
η2nd Law =
w
62.1
=
= 0.725
ϕ1-ϕ2 85.7
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.146
A 10- m3 storage tank contains methane at low temperature. The pressure inside is
700 kPa, and the tank contains 25% liquid and 75% vapor, on a volume basis. The
tank warms very slowly because heat is transferred from the ambient.
a. What is the temperature of the methane when the pressure reaches 10 MPa?
b. Calculate the heat transferred in the process, using the generalized charts.
c. Repeat parts (a) and (b), using the methane tables, Table B.7. Discuss the
differences in the results.
CH4: V = 10 m3, P1 = 700 kPa
VLIQ 1 = 2.5 m3, VVAP 1 = 7.5 m3
0.70
10
a) Pr1 = 4.60 = 0.152, Pr2 = 4.60 = 2.174
From D.1: ZF1 = 0.025, ZG1 = 0.87 &
T1 = 0.74 × 190.4 = 140.9 K
vF1 =
0.025×0.5183×140.9
= 0.00261
700
0.87×0.5183×140.9
= 0.0908
700
2.5
7.5
mLIQ 1 = 0.00261 = 957.9 kg, mVAP 1 = 0.0908 = 82.6 kg
Total m = 1040.3 kg
Z2×0.5183×190.4×Tr2
V
10
v2 = v1 = m = 1040.5 = 0.00961 =
10 000
vG1 =
or Z2Tr2 = 0.9737 at Pr2 = 2.174
By trial and error
Tr2 = 1.334 & Z2 = 0.73,
T2 = 1.334×190.4 = 254.0 K
b) Energy Eq.:
Q12 = m(u2-u1) = m(h2-h1) - V(P2-P1)
82.6
Using D.2 & x1 = 1040.5 = 0.0794
*
*
(h1-h1) = (h1-hf1) - x1hfg1
[
] = 431.1
= 0.518 35×190.4 4.72-0.0794(4.72-0.29)
*
*
(h2 - h1) = CP (T2 – T1) = 2.254 (254.0 - 140.9) = 254.9
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
*
(h2 - h2) = 0.5183 × 190.4(1.47) = 145.1
(h2 - h1) = -145.1 + 254.9 + 431.1 = 540.9 kJ/kg
Q12 = 1040.5 kg (540.9) kJ/kg – 10 m3 (10 000-700) kPa
= 469 806 kJ
c) Using Table B.7 for CH4
T1 = TSAT 1 = 141.7 K,
vF1 = 0.002 675,
uF1 = -178.47
vG1 = 0.090 45 , uG1 = 199.84
2.5
mLIQ 1 = 0.002 675 = 934.6,
Total mass
7.5
mVAP 1 = 0.090 45 = 82.9
m = 1017.5 kg and
10
v2 = 1017.5 = 0.009 828 m3/kg
T2 = 259.1 K
At v2 & P2 = 10 MPa → u = 296.11
2
Q12 = m(u2-u1) = 1017.5×296.11 - 934.6(-178.47) - 82.9(199.84)
= 451 523 kJ
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.147
Consider the following reference state conditions: the entropy of real saturated
liquid methane at −100°C is to be taken as 100 kJ/kmol K, and the entropy of
hypothetical ideal gas ethane at −100°C is to be taken as 200 kJ/kmol K.
Calculate the entropy per kmol of a real gas mixture of 50% methane, 50% ethane
(mole basis) at 20°C, 4 MPa, in terms of the specified reference state values, and
assuming Kay’s rule for the real mixture behavior.
CH : T = -100 oC, s= 100 kJ/kmol K
4
0
LIQ 0
C2H6: T0 = -100 oC, P0 = 1 MPa,
*
s-0 = 200 kJ/kmol K
Also for CH4: TC = 190.4 K, PC = 4.60 MPa
For a 50% mixture Kay’s rule Eq.12.84:
Tcmix = 0.5 × 190.4 + 0.5 × 305.4 = 247.9 K
Pcmix = 0.5 × 4.60 + 0.5 × 4.88 = 4.74 MPa
IG MIX at T0(=-100 oC), P0(=1 MPa):
CH4: Tr0 = 0.91 ,
PG = 0.57 × 4.60 = 2.622 MPa
*
s-0 CH4 = s-LIQ 0 P + (s-*-s-LIQ)at P - R ln (P0/PG)
G
G
= 100 + 4.01×8.3145 - 8.3145 ln (1/2.622) = 141.36
*
s-0 MIX = 0.5×141.36 + 0.5×200 - 8.3145(0.5 ln 0.5 + 0.5 ln 0.5) = 176.44
CP0 MIX = 0.5×16.04×2.254 + 0.5×30.07×1.766 = 44.629
293.2
4
*
s-TP MIX = 176.44 + 44.629 ln 173.2 - 8.3145 ln 1 = 188.41 kJ/kmol K
For the mixture at T, P: Tr = 1.183,
Entropy departure
Pr = 0.844
*
s-TP MIX - s-TP MIX = 0.4363×8.3145 = 3.63 kJ/kmol K
Therefore,
s-TP MIX = 188.41 - 3.63 = 184.78 kJ/kmol K
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
An alternative is to form the ideal gas mixture at T, P instead of at T0, P0 :
T P
*
s-TP CH4 = s-LIQ 0 + (s-*-s-LIQ) + CP0 CH4 ln T - R ln P
0
P ,T
G 0
G
at P , T
G 0
293.2
4
= 100 + 33.34 + 16.04×2.254 ln 173.2 - 8.3145 ln 2.6
= 100 + 33.34 + 19.03 - 3.53 = 148.84 kJ/kmol K
293.2
4
*
s-TP C2H6 = 200 + 30.07×1.766 ln 173.2 - 8.3145 ln 1
= 200 + 27.96 - 11.53 = 216.43 kJ/kmol K
*
s= 0.5×148.84 + 0.5×216.43
TP MIX
- 8.3145(0.5 ln 0.5 + 0.5 ln 0.5) = 188.41 kJ/kmol K
s-TP MIX = 188.41 - 3.63 = 184.78 kJ/kmol K
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.148
Determine the heat transfer and the net entropy change in problem 12.129. Use
the initial pressure of the carbon dioxide to be 4.56 MPa before the ethylene is
flowing into the tank.
A gas mixture of a known composition is frequently required for different
purposes, e.g., in the calibration of gas analyzers. It is desired to prepare a gas
mixture of 80% ethylene and 20% carbon dioxide (mole basis) at 10 MPa, 25°C
in an uninsulated, rigid 50-L tank. The tank is initially to contain CO2 at 25°C
and some pressure P1. The valve to a line flowing C2H4 at 25°C, 10 MPa, is now
opened slightly, and remains open until the tank reaches 10 MPa, at which point
the temperature can be assumed to be 25°C. Assume that the gas mixture so
prepared can be represented by Kay’s rule and the generalized charts. Given the
desired final state, what is the initial pressure of the carbon dioxide, P1?
A = C2H4, B = CO2
Pi =10 MPaA
o
Ti = 25 C
T1 = 25 oC
P2 = 10 MPa, T2 = 25 oC
V=0.05 3
m B
yA2 = 0.8, yB2 = 0.2
Mixture at 2 :
PC2 = 0.8 × 5.04 + 0.2 × 7.38 = 5.508 MPa
TC2 = 0.8 × 282.4 + 0.2 × 304.1 = 286.7 K
Tr2 = 298.15/286.7 = 1.040; Pr2 = 10/5.508 = 1.816
D.1 :
n2 =
Z2 = 0.32
P2V
10 000×0.05
- = 0.32×8.3145×298.2 = 0.6302 kmol
Z2RT2
nA2 = ni = 0.8 n2 = 0.5042 kmol C2H4
nB2 = n1 = 0.2 n2 = 0.1260 kmol CO2
298.2
Tr1 = 304.1 = 0.981 and
4560
Pr1 = 7380 = 0.618
Energy Eq.: QCV + nihi = n2u-2 - n1u-1 = n2h2 - n1h1 - (P2-P1)V
- -*
- -*
- -*
or QCV = n2(h2-h2) - n1(h1-h1) - ni(hi-hi ) - (P2-P1)V
-* -* -*
(since Ti = T1 = T2, hi = h1 = h2)
-* (h1-h1) = 0.83 × 8.3145 × 304.1 = 2099 kJ/kmol
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
-* (h2-h2) = 3.40 × 8.3145 × 286.7 = 8105 kJ/kmol
298.2
10
Tri = 282.4 = 1.056, Pri = 5.04 = 1.984
-* (hi -hi) = 3.35×8.3145×282.4 = 7866 kJ/kmol
QCV = 0.6302(-8105) - 0.126(-2099) - 0.5042(-7866) - (10 000-4560)×0.05
= -1149 kJ
∆SCV = n2s-2 - n1s-1 , ∆SSURR = - QCV/T0 - nis-i
∆Sgen = n2s-2 - n1s-1 - QCV/T0 - nis-i
*
*
Let s-A0 = s-B0 = 0 at T0 = 25 oC, P0 = 0.1 MPa
*
Then s-MIX 0 = -8.3145 (0.8 ln 0.8 + 0.2 ln 0.2) = 4.161 kJ/kmol K
*
*
*
*
s-1 = s-B0 + (s-P1 T1-s-P0 T0)B + (s-1-s-P1 T1)B
4.56
= 0 + (0-8.3145 ln 0.1 ) - 0.60 × 8.3145 = -36.75 kJ/kmol K
*
*
*
*
s-i = s-A0 + (s-Pi Ti-s-P0 T0)A + (s-i-s-Pi Ti)A
10
= 0 + (0-8.3145 ln 0.1) - 2.44×8.3145 = -58.58 kJ/kmol K
*
*
*
*
s-2 = s-MIX 0 + (s-P2 T2-s-P0 T0)MIX + (s-2-s-P2 T2)MIX
10
= 4.161 + (0-8.3145 ln 0.1) - 2.551×8.3145 = -55.34 kJ/kmol K
Sgen = 0.6302(-55.33) - 0.126(-36.75) - 0.5042(-58.58) + 1149/298.2
= +3.15 kJ/K
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke Sonntag
Fundamentals of
Thermodynamics
SOLUTION MANUAL
CHAPTER 12
English Units
8e
UPDATED JULY 2013
Borgnakke and Sonntag
CHAPTER 12
CONTENT CHAPTER 12
SUBSECTION
PROB NO.
Clapeyron equation
Volume Expansivity and Compressibility
Equations of State
Generalized Charts
Mixtures
Review problem
149-153
154-160
161-162
163-178
179-181
182
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
Clapeyron Equation
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.149E
Verify that Clapeyrons equation is satisfied for R-410A at 30 F in Table F.9.
Clapeyron Eq.:
F.9:
P = 111.796 psia,
dPsat dPg hfg
dT = dT = Tvfg
hfg = 95.75 Btu/lbm,
vfg = 0.5289 ft3/lbm
Slope around 30 F best approximated by cord from 20 F to 40 F
dPg 133.163 – 93.128
= 2.00 psia/R,
dT =
40 – 20
hfg
778
95.75
=
Tvfg 489.7 × 0.5289 144 = 1.997 psia/R
This fits very well.
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.150E
Use the approximation given in Problem 12.16 and Table F.7 to determine A and
B for steam from properties at 70 F only. Use the equation to predict the
saturation pressure at 80 F and compare to table value.
dPsat
-2
dT = Psat (-B)(-T )
so we notice from Eq.12.7 and Table values from F.7.1 and F.4 that
hfg
1053.95
B = R = 85.76 / 778 = 9561.3 R
Now the constant A comes from the saturation pressure as
9561.3
A = ln Psat + B/T = ln 0.363 + 459.67 + 70 = 17.038
Use the equation to predict the saturation pressure at 80 F as
9561.3
ln Psat = A – B/T = 17.038 - 459.67 + 80 = -0.6789
Psat = 0.5071 psia
compare this with the table value of Psat = 0.507 psia and we have a very accurate
approximation.
ln Psat = A – B/T
⇒
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.151E
Find the saturation pressure for refrigerant R-410A at –100 F assuming it is higher
than the triple point temperature.
The lowest temperature in Table F.9 for R-410A is -80 F, so it must be
extended to -100 F using the Clapeyron Eq. 12.7 integrated as in example 12.1
At T1 = -80 F = 379.7 R ,
P1 = 8.196 lbf/in.2
and
1.9859
R = 72.585 = 0.027 36 Btu/lbm R
P hfg (T-T1) 121.14 (359.7-379.7)
=
= -0.6484
ln P = R
T × T1 0.027 36 359.7 × 379.7
1
P = P1 exp(-0.6484) = 4.286 lbf/in.2
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.152E
Using thermodynamic data for water from Tables F.7.1 and F.7.3, estimate the
freezing temperature of liquid water at a pressure of 5000 lbf/in.2.
P
5000 psia
dPif hif
H2O dT = Tv ≈ constant
if
At the triple point,
T.P.
vif = vf - vi = 0.016 022 - 0.017 473
T
= -0.001 451 ft3/lbm
hif = hf - hi = 0.0 - (-143.34) = 143.34 Btu/lbm
dPif
143.34
778.2
2
=
×
dT 491.69(-0.001 451) 144 = -1085.8 lbf/in. R
⇒
at P = 5000 lbf/in2,
(5000-0.09)
T ≈ 32.02 + (-1085.8) = 27.4 F
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.153E
Ice (solid water) at 27 F, 1 atm is compressed isothermally until it becomes liquid.
Find the required pressure.
Water, triple point T = 32.02 F = 491.69 R,
vf = 0.016 022 ft3/lbm,
vi = 0.017 473 ft3/lbm
hf = 0.00 Btu/lbm
Clapeyron
P = 0.088 67 lbf/in.2
hi = -143.34 Btu/lbm
dPif
hf - hi
143.34×778.2
=
=
dT (vf - vi)T -0.001 451×491.69×144 = -1085.8 psia/R
dPif
∆P ≈ dT ∆T = -1085.8 (27 - 32.02) = 5450.7 lbf/in.2
P = Ptp + ∆P = 5451 lbf/in.2
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
Volume Expansivity and Compressibility
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.154E
Determine the volume expansivity, αP, and the isothermal compressibility, βT, for
water at 50 F, 500 lbf/in.2 and at 400 F, 1500 lbf/in.2 using the steam tables.
Water at 50 F, 500 lbf/in.2 (compressed liquid)
1 ∂v
1 ∆v
αP = v( )P ≈ v( )P
∂T
∆T
Using values at 32 F, 50 F and 100 F
1
0.016 1 - 0.015 99
αP ≈ 0.015 99
= 0.000 101 F-1
100 - 32
1 ∂v
1 ∆v
βT = - v( )T ≈ - v( )T
∂P
∆P
Using values at saturation, 500 and 1000 lbf/in.2
1
0.015 99 - 0.016 02
βT ≈ - 0.015 99
= 0.000 0019 in.2/lbf
1000 - 0.178
Water at 400 F, 1500 lbf/in.2 (compressed liquid)
1
0.019 25 - 0.017 85
= 0.000 76 F-1
αP ≈ 0.018 45
450 - 350
1
0.0184 - 0.0185
βT ≈ - 0.018 45 2000 - 1000 = 0.000 0054 in.2/lbf
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.155E
Use the CATT3 software to solve the previous problem.
The benefit of the software to solve for the partial derivatives is that we can
narrow the interval over which we determine the slope.
Water at 50 F, 500 lbf/in.2 (compressed liquid)
1 ∂v
1 ∆v
1 ∂v
1 ∆v
αP = v( )P ≈ v( )P ; βT = - v( )T ≈ - v( )T
∂T
∆T
∂P
∆P
Estimate by finite difference using values at 40 F, 50 F and 60 F,
1
0.01601 - 0.01599
= 0.000 0625 F-1
αP ≈ 0.01600
60 - 40
Using values at saturation, 500 psia and 1000 psia,
1 0.01597 - 0.01602
βT ≈ - 0.0160 1000 - 0.178 = 0.000 0031 psi-1
Water at 400 F, 1500 lbf/in.2 (compressed liquid)
Estimate by finite difference using values at 350 F, 400 F and 450 F,
1
0.019 26 - 0.017 86
αP ≈ 0.01 849
= 0.000 757 F-1
450 - 350
Using values at 1000 psia, 1500 psia and 2000 psia,
1
0.01 844 - 0.01 855
βT ≈ - 0.018 49
= 0.000 0059 psi-1
2000 - 1000
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.156E
A cylinder fitted with a piston contains liquid methanol at 70 F, 15 lbf/in.2 and
volume 1 ft3. The piston is moved, compressing the methanol to 3000 lbf/in.2 at
constant temperature. Calculate the work required for this process. The isothermal
compressibility of liquid methanol at 70 F is 8.3 × 10−6 in.2/lbf.
2
2
⌠ ∂v
⌠
w
=
Pdv
=
P
dP
=
vβ PdPT
1 2 ⌡
⌡ T
∂P T T ⌠
⌡
1
( )
1
For v ≈ const & βT ≈ const.
=>
vβT 2 2
w
=
1 2
2 P2 - P1
(
)
For liquid methanol, from Table F.3 : ρ = 49.1 lbm/ft3
V1 = 1.0 ft3,
W =
1 2
m = 1.0 × 49.1 = 49.1 lbm
1.0 × 8.3 × 10−6
(3000)2 - (15)2 ×144 = 5378.4 ft lbf = 6.9 Btu
2
[
]
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.157E
Sound waves propagate through a media as pressure waves that causes the media
to go through isentropic compression and expansion processes. The speed of
sound c is defined by c2 = (∂P/∂ρ)s and it can be related to the adiabatic
compressibility, which for liquid ethanol at 70 F is 6.4 × 10-6 in.2/lbf. Find the
speed of sound at this temperature.
c2 =
∂P
1
1
(∂P
)
=
-v
(
)
=
=
∂ρ s
∂v s 1 ∂v
βρ
-v( )s ρ
∂P
2
s
From Table F.3 for ethanol,
⇒c=
ρ = 48.9 lbm/ft3
32.174×144
(6.4×10
) = 3848 ft/s
×48.9
1/2
-6
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.158E
Consider the speed of sound as defined in Eq. 12.42. Calculate the speed of sound
for liquid water at 50 F, 250 lbf/in.2 and for water vapor at 400 F, 80 lbf/in.2 using
the steam tables.
From Eq 12.42 :
c2 =
∂P
(∂P
)
=
-v
(
)
∂ρ s
∂v s
2
Liquid water at 50 F, 250 lbf/in.2
Assume
(∂P
) ≈ (∆P
)
∂v s
∆v
T
Using saturated liquid at 50 F and compressed liquid at 50 F, 500 lbf/in.2,
(0.016024+0.015998
) ((500-0.18)×144×32.174
2
0.015998-0.016024 ) = 22.832×10
2
c2 = -
6
c = 4778 ft/s
Superheated vapor water at 400 F, 80 lbf/in.2
v = 6.217 ft3/lbm,
s = 1.6790 Btu/lbm R
At P = 60 lbf/in.2 & s = 1.6790: T = 343.8 F, v = 7.7471 ft3/lbm
At P = 100 lbf/in.2 & s = 1.6790: T = 446.2 F, v = 5.2394 ft3/lbm
c2 = -(6.217)2
((100-60)×144×32.174
) = 2.856 × 10
5.2394-7.7471
6
c = 1690 ft/s
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.159E
Liquid methanol at 77 F has an adiabatic compressibility of 7.1 × 10-6 in2/lbf.
What is the speed of sound? If it is compressed from 15 psia to 1500 psia in an
insulated piston/cylinder, what is the specific work?
From Eq.12.41 and Eq.12.42 and the density from table F.3
c2 =
(∂P
) = −v (∂P
) = β1ρ = 7.1 × 101-6 × 49.1 144 × 32.174 ft2/s2
∂ρ s
∂v s
2
s
= 13.290 × 106 ft2/s2
c = 3645 ft/s
The specific work becomes
2
w = ⌠P
⌡ dv = ⌠
⌡ P dP
⌡P (-βsv ) dP = − ⌠
⌡ βsv P dP = −βs v ⌠
1
2
2
= −βs v 0.5 (P2 – P1)
0.5
= −7.1 × 10-6 in2/lbf × 49.1 ft3/lbm × (15002 – 152) psi2
= −0.163 (ft-lbf/lbm) (ft/in)2 = −23.4 ft-lbf/lbm = -0.03 Btu/lbm
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
Equations of State
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.160E
Use Table F.9 to find the compressibility of R-410A at 140 F and a) saturated
liquid b) saturated vapor and c) 400 psia
.
Table F.1:
a)
R = 1545.36 / 72.585 = 21.29 lbf-ft/lbm-R
Table F.9.1: P = 556.488 psia, v = 0.01966 ft3/lbm
Pv 556.488 × 0.01966 × 144
= 0.1234
Z = RT =
21.29 × 599.7
b)
Table F.9.1: P = 556.488 psia, v = 0.0796 ft3/lbm
Pv 556.488 × 0.0796 × 144
= 0.5
Z = RT =
21.29 × 599.7
c)
Table F.9.2: P = 400 psia, v = 0.1574 ft3/lbm
Pv 400 × 0.1574 × 144
Z = RT = 21.29 × 599.7 = 0.71
The R-410A is not an ideal gas at any of these states.
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.161E
Calculate the difference in internal energy of the ideal-gas value and the real-gas
value for carbon dioxide at the state 70 F, 150 lbf/in.2, as determined using the
virial EOS. At this state B = -2.036 ft3/lb mol, T(dB/dT) = 4.236 ft3/lb mol.
Solution:
virial:
CO2 at 70 F, 150 lbf/in2
∂P
RT dB
(∂T
)v = Rv + BR
+
( )
v dT
v
RT BRT
P= v + 2 ;
v
2
2
⌠∞ RT2 dB
⌠∞
∂P
RT2 dB
u-u = - T
dv = - v dT
- P dv = -
2
∂T v
⌡v
⌡v v dT
*
[( ) ]
B = -2.036 ft3/lbmol
( )
dB
T dT = 4.236 ft3/lbmol
( )
Solution of virial equation (quadratic formula):
-v = 1 RT 1 +
2 P
[
But
]
1+4BP/RT
RT 1545×529.7
P = 150×144 = 37.8883
[
] = 35.7294 ft /lbmol
v- = 0.5×37.8883 1 + 1+4(-2.036)/37.8883
3
Using the minus-sign root of the quadratic formula results in a compressibility
factor < 0.5, which is not consistent with such a truncated equation of state.
RT dB -1.9859×529.7
u- - u-* = - v T dT =
4.236 = -123.9 Btu/lbmol
35.7294
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
Generalized Charts
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.162E
How low should the pressure be so that nitrous oxide (N2O) gas at 501.6 R can be
treated as an ideal gas with 5% accuracy or better?
From Table F.1:
Tc = 557.3 R,
Pc = 1050 psi
501.6
=> Tr1 = 557.3 = 0.9
Look in Fig. D.1 following the curve Tr1 = 0.9 to the point where Z = 0.95
Pr1 = 0.125
so
P < 0.125 × 1050 psi = 131 psi
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.163E
Nitrous oxide (N2O) at 501.6 R is at a pressure so that it can be in a two-phase
state. Find the generalized enthalpy departure for the two saturated states of liquid
and vapor.
From Table F.1:
Tc = 557.3 R,
Pc = 1050 psi
501.6
=> Tr1 = 557.3 = 0.9
From Fig. D.2:
Saturated liquid:
(h*- hf)/RTc = 4.08;
Saturated vapor:
(h*- hg)/RTc = 0.875
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.164E
Find the heat of evaporation, hfg, for R-134a at 30 F from the generalized charts
and compare to the value in Table F.10.
From Table F.1:
Tc = 673.6 R,
489.7
Pc = 589 psia => Tr1 = 673.6 = 0.727
R = 1.98589/102.03 = 0.01946 Btu/lbm-R
From Fig. D.2:
Saturated liquid:
(h*- hf)/RTc = 4.7;
Saturated vapor:
(h*- hg)/RTc = 0.3
hfg = hg - hf = RTc [-0.3 – (-4.7)] = 4.4 RTc
= 4.4 × 0.01946 × 673.6 = 57.67 Btu/lbm
Table F. 10:
hfg = 85.63 Btu/lbm
The approximation is not very good and can be improved by using the
accentric factor.
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.165E
A 7-ft3 rigid tank contains propane at 1300 lbf/in.2, 540 F. The propane is then
allowed to cool to 120 F as heat is transferred with the surroundings. Determine
the quality at the final state and the mass of liquid in the tank, using the
generalized compressibility charts.
Propane C3H8:
V = 7.0 ft3, P1 = 1300 lbf/in.2, T1 = 540 F = 1000 R
cool to T2 = 120 F = 580 R
From Table F.1 : TC = 665.6 R, PC = 616 lbf/in.2
1300
1000
Pr1 = 616 = 2.110, Tr1 = 665.6 = 1.502
From D.1: Z1 = 0.83
v2 = v1 =
Z1RT1
P1
=
0.83 × 35.04 × 1000
= 0.1554 ft3/lbm
1300 × 144
From D.1 at Tr2 = 0.871, saturated => PG2 = 0.43 × 616 = 265 lbf/in.2
vG2 =
0.715 × 35.04 × 580
= 0.3808 ft3/lbm
265 × 144
vF2 =
0.075 × 35.04 × 580
= 0.0399 ft3/lbm
265 × 144
0.1554 = 0.0399 + x2(0.3781-0.0399)
=>
x2 = 0.3388
7.0
mLIQ 2 = (1 - 0.3388) 0.1554 = 29.8 lbm
These tanks
contain liquid
propane.
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.166E
A rigid tank contains 5 lbm of ethylene at 450 lbf/in.2, 90 F. It is cooled until the
ethylene reaches the saturated vapor curve. What is the final temperature?
C2H4 m = 5 lbm
C2 H4
P1 = 450 lbf/in2, T1 = 90 F = 249.7 R
T
1
450
Pr1 = 731 = 0.616,
2
Fig. D.1
⇒
v
Z2Tr2
ZG2Tr2
1 r1
0.82 × 1.082
Pr2 = Pr1 Z T = 0.616
549.7
Tr1 = 508.3 = 1.082
Z1 = 0.82
= 0.6943 ZG2Tr2
Trial & error to match a saturated Pr2, Tr2 and the ZG2 so Eq. is satisfied.
Guess a Tr2 and find the rest and compare with computed Pr2 from Eq.
Tr2
ZG2
Pr2
Pr2 CALC
0.871 0.715
0.43
0.432
~ OK
=> T2 = 442.7 R
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.167E
A piston/cylinder contains 10 lbm of butane gas at 900 R, 750 lbf/in.2. The butane
expands in a reversible polytropic process with polytropic exponent, n = 1.05,
until the final pressure is 450 lbf/in.2. Determine the final temperature and the
work done during the process.
C4H10 ,
P1 = 750 lbf/in.2
m = 10 lbm , T1 = 900 R ,
Rev. polytropic process (n = 1.05), P2 = 450 lbf/in.2
900
750
Tr1 = 765.4 = 1.176, Pr1 = 551 = 1.361
V1 = mZRT/P =
=> From Fig. D.1 :
Z1 = 0.67
10 × 0.67 × 26.58 × 900
= 1.484 ft3
750 × 144
n
n
P1V1 = P2V2 → V2 = 1.484
1
750 1.05
= 2.414 ft3
450
( )
P2V2 450 × 144 × 2.414
Z2Tr2 = mRT =
= 0.7688
C 10 × 26.58 × 765.4
at Pr2 = 450/551 = 0.817
Trial & error:
Tr2 = 1.068,
2
P2V2 - P1V1
W = ⌠ PdV =
1 2 ⌡
1
1-n
=
Z2 = 0.72
=>
T2 = 817.4 R
- 750 × 1.484
(450 × 2.414
) × 144
778 = 98.8 Btu
1 - 1.05
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.168E
Calculate the heat transfer during the process described in Problem 12.167E.
From solution 12.167,
V1 = 1.473 ft3,
V2 = 2.396 ft3,
Tr1 = 1.176,
Pr1 = 1.361,
From D.1:
h*-h
RTC 1 = 1.36,
*
( )
1
W2 = 98.8 Btu
Tr2 = 1.068,
Pr2 = 0.817, T2 = 817.4 R
h*-h
RTC 2 = 0.95
( )
*
h2 - h1 = 0.415 (817.4 - 900) = -34.3 Btu/lbm
h2 - h1 = -34.3 +
26.58×765.4
(-0.95 + 1.36) = -23.6 Btu/lbm
778
U2 - U1 = m(h2 - h1) - P2V2 + P1V1
= 10(-23.6) -
450×144×2.414 750×144×1.484
+
= -231.1 Btu
778
778
Q = U2 - U1 + 1W2 = -132.3 Btu
1 2
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.169E
The new refrigerant R-152a is used in a refrigerator with an evaporator
temperature of -10 F and a condensing temperature of 90 F. What are the high and
low pressures in this cycle?
Since we do not have the printed tables for R-152a we will use generalized charts.
The critical properties are: Tc = 695.5 R, Pc = 656 psi.
Tr1 = T/Tc = (459.67 – 10)/ 695.5 = 0.646
Fig. D.1:
PG T1 = Pr1 sat Pc = 0.06 × 656 = 39.4 psi
Tr2 = T/Tc = (459.67 + 90)/ 695.5 = 0.79
Fig. D.1:
PG T2 = Pr2 sat Pc = 0.25 × 656 = 164 psi
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.170E
A cylinder contains ethylene, C2H4, at 222.6 lbf/in.2, 8 F. It is now compressed in
a reversible isobaric (constant P) process to saturated liquid. Find the specific
work and heat transfer.
Ethylene C2H4 P1 = 222.6 lbf/in.2 = P2, T1 = 8 F = 467.7 R
State 2: saturated liquid, x2 = 0.0
R = 55.07 ft lbf/lbm R = 0.070 78 Btu/lbm R
467.7
222.6
Tr1 = 508.3 = 0.920 Pr1 = Pr2 = 731 = 0.305
*
Z1 = 0.85 ,
From D.1 and D.2:
v1 =
Z1RT1
P1
=
h -h
(RT
) = 0.40
C
1
0.85 × 55.07 × 467.7
= 0.683
222.6 × 144
*
(h1-h1) = 0.070 78 × 508.3 × 0.40 = 14.4
From D.1 and D.2:
Z2 = 0.05 ,
v2 =
Z2RT2
P2
T2 = 0.822 × 508.3 = 417.8 R
h*-h
RTC 2 = 4.42
( )
=
0.05 × 55.07 × 417.8
= 0.035 89 ft3/lbm
222.6 × 144
*
(h2-h2) = 0.070 78 × 508.3 × 4.42 = 159.0 Btu/lbm
*
*
(h2-h1) = CP0(T2-T1) = 0.411(417.8 - 467.7) = -20.5 Btu/lbm
2
144
⌠ Pdv = P(v2-v1) = 222.6(0.035 89 - 0.683) ×
w =⌡
778 = -26.7 Btu/lbm
1 2
1
q = (u2 - u1) + 1w2 = (h2-h1) = -159.0 - 20.5 + 14.4 = -165.1 Btu/lbm
1 2
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.171E
Saturated vapor R-410A at 80 F is throttled to 30 lbf/in.2 in a steady flow process.
Find the exit temperature, neglecting kinetic energy, using the generalized charts,
Fig. D.2 and repeat using Table F.9.
R-410A throttling process, R = 1.98589/72.585 = 0.02736 Btu/lbm-R
T1 = 80 F, x1 = 1.00, P2 = 30 lbf/in.2,
*
Energy Eq.:
*
*
*
h2-h1 = (h2-h2) + (h2-h1) + (h1-h1) = 0
Generalized charts,
From D.2:
539.7
Tr1 = 620.1 = 0.87
*
(h1-h1) = 0.02736 × 620.1 × 0.75 = 12.72 Btu/lbm
To get CP0, use h values from Table F.9 at low pressure (5 psia).
CP0 ≈
137.6-133.75
= 0.1925 Btu/lbm R
80-60
Substituting into energy Eq.:
*
(h2-h2) + 0.1925 (T2 - 30) + 12.72 = 0
30
at Pr2 = 711 = 0.042
469.7
Assume T2 = 10 F = 469.7 R => Tr2 = 620.1 = 0.757
*
(h2-h2) = 0.02736 × 620.1 × 0.07 = 1.19
Substituting,
-1.19 + 0.1925(10 - 80) + 12.72 = -1.945
479.7
Guess T2 = 20 F = 479.7 R => Tr2 = 620.1 = 0.774
*
(h2-h2) = 0.02736 × 620.1 × 0.07 = 1.19
Substituting,
-1.19 + 0.1925(20 - 80) + 12.72 = -0.02 nearly OK
⇒ T2 = 20 F (maybe re-estimate CP0 at avg T)
R-410A tables, F.9:
T1 = 80 F, x1 = 1.0
=> h1 = 122.14 Btu/lbm
h2 = h1 = 122.14 Btu/lbm, P2 = 30 lbf/in.2 => T2 = 8.15 F
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.172E
Find the heat of evaporation, hfg, for isobutane (Tc = 735.8 R, Pc = 529.4
psia, M = 58.124) at 54.7 F from the generalized charts and compare to the values
in the CATT3 computerized tables.
To read the charts we need the reduced temperature
Tr1 = T/Tc = (54.7 + 459.7)/735.8 = 0.70
R = 1.98589 / 58.124 = 0.03417 Btu/lbm-R
From Fig. D.2:
(h*− h)g = 0.2 RTc ; (h*− h)f = 4.85 RTc
hfg = hg – hf = − (h*− h)g − (h*− h)f = (−0.2 + 4.85) RTc
= 4.65 RTc = 4.65 × 0.03417 Btu/lbm-R × 735.8 R
= 116.9 kJ/kg
CATT3:
hfg = –11.01 – (–158.5) = 147.5 Btu/lbm
The generalized charts are not super accurate, some improvement can be
done using the accentric factor.
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.173E
A 10-ft3 tank contains propane at 90 F, 90% quality. The tank is heated to 600 F.
Calculate the heat transfer during the process.
P
V = 10 ft3
T1 = 90 F = 549.7 R, x1 = 0.90
2
Heat to T2 = 600 F = 1059.7 R
M = 44.094, TC = 665.6 R
1
PC = 616 lbf/in.2
V
R = 35.04, CP0 = 0.407 Btu/lbm R
Tr1 = 0.826 Figs. D.1 and D.2 →
Z1 = 0.1 × 0.053 + 0.9 × 0.78 = 0.707,
*
h1-h1
RTc = 0.1 × 4.4 + 0.9 × 0.55 = 0.935
SAT
Pr
= 0.31 ;
PV
m = ZRT =
Pr2 =
SAT
P1
= 0.31 × 616 = 191 lbf/in.2
191 × 144 × 10
= 20.2 lbm
0.707 × 35.04 × 549.7
20.2 × Z2 × 35.04 × 1059.7
616 × 144 × 10
Z2
= 1.183
at Tr2 = 1.592
Pr2 = 0.79 and P2 = 490 lbf/in.
*
h2-h2
Trial & error:
Z2 = 0.94 and RTc = 0.36
2
*
*
(h2-h1) = 0.407(600-90) = 207.6 Btu/lbm
*
(h1-h1) = 0.935 × 35.04 × 665.9/ 778 = 28.0
*
(h2-h2) = 0.36 × 35.04 × 665.9/ 778 = 10.8
From the energy equation
Q12 = m(h2-h1) - (P2-P1)V
= 20.2 (-10.8 + 207.6 + 28.0) - (490 - 191) ×
144 × 10
778
= +4541 - 553 = 3988 Btu
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.174E
Carbon dioxide collected from a fermentation process at 40 F, 15 lbf/in.2 should
be brought to 438 R, 590 lbf/in.2 in a steady flow process. Find the minimum
amount of work required and the heat transfer. What devices are needed to
accomplish this change of state?
35.1
R = 778 = 0.045 12 Btu/lbm R
500
Tri = 547.4 = 0.913 ,
15
Pri = 1070 = 0.014
*
*
h -h
(RT
) = 0.02, (sR-s) = 0.01 R
From D.2 and D.3:
C
438
Tre = 547.4 = 0.80,
From D.2 and D.3:
*
590
Pre = 1070 = 0.551
*
he-he = 4.50 RTc,
*
*
*
se-se = 4.70 R
*
(hi-he) = - (hi -hi) + (hi -he) + (he-he)
= - 0.045 12 × 547.4 × 0.02 + 0.203(500 - 438) + 0.045 12 × 547.4 × 4.50
= 123.2 Btu/lbm
*
* *
*
(si-se) = - (si -si) + (si -se) + (se-se)
500
15
= - 0.045 12×0.01 + 0.203 ln 438 - 0.045 12 ln 590 + 0.045 12×4.70
= 0.4042 Btu/lbm R
wrev = (hi-he) -T0(si-se) = 123.2 - 500(0.4042) = -78.4 Btu/lbm
qrev = (he-hi) + wrev = -123.2 - 78.9 = -202.1 Btu/lbm
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.175E
A cylinder contains ethylene, C2H4, at 222.6 lbf/in.2, 8 F. It is now compressed
isothermally in a reversible process to 742 lbf/in.2. Find the specific work and
heat transfer.
Ethylene C2H4 ,
R = 55.07 ft lbf/lbm R = 0.070 78 Btu/lbm R
State 1: P1 = 222.6 lbf/in.2,
T2 = T1 = 8 F = 467.7 R
State 2: P2 = 742 lbf/in.2
467.7
Tr2 = Tr1 = 508.3 = 0.920;
222.6
Pr1 = 731 = 0.305
*
From D.1, D.2 and D.3:
Z1 = 0.85 ,
h -h
(RT
) = 0.40
C
1
*
(h1-h1) = 0.070 78 × 508.3 × 0.40 = 14.4 Btu/lbm
*
(s1-s1) = 0.070 78 × 0.30
= 0.0212 Btu/lbm R
742
Pr2 = 731 = 1.015 (comp. liquid)
From D.1, D.2 and D.3:
Z2 = 0.17
*
(h2-h2) = 0.070 78 × 508.3 × 4.0 = 143.9
*
(s2-s2) = 0.070 78 × 3.6 = 0.2548
*
*
(h2-h1) = 0
742
* *
(s2-s1) = 0 - 0.070 78 ln 222.6 = -0.0852
q = T(s2-s1) = 467.7(-0.2548 - 0.0852 + 0.0212) = -149.1 Btu/lbm
1 2
(h2-h1) = -143.9 + 0 + 14.4 = -129.5
(u2-u1) = (h2-h1) - RT(Z2-Z1)
= -129.5 - 0.070 78 × 467.7 (0.17 - 0.85) = -107.0
w = 1q2 - (u2-u1) = -149.1 + 107.0 = -42.1 Btu/lbm
1 2
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.176E
A geothermal power plant on the Raft river uses isobutane as the working fluid.
The fluid enters the reversible adiabatic turbine at 320 F, 805 lbf/in.2 and the
condenser exit condition is saturated liquid at 91 F. Isobutane has the properties
Tc = 734.65 R, Pc = 537 lbf/in.2, Cpo = 0.3974 Btu/lbm R and ratio of specific
heats k = 1.094 with a molecular weight as 58.124. Find the specific turbine work
and the specific pump work.
R = 26.58 ft lbf/lbm R = 0.034 166 Btu/lbm R
Turbine inlet: Tr1 = 779.7 / 734.7 = 1.061,
Condenser exit: T3 = 91 F , x3 = 0.0 ;
Pr1 = 805 / 537 = 1.499
Tr3 = 550.7 / 734.7 = 0.75
Pr3 = 0.165, Z3 = 0.0275
From D.1 :
P2 = P3 = 0.165 × 537 = 88.6 lbf/in.2
From D.2 and D.3,
*
(h1-h1) = 0.034 166 × 734.7 × 2.85 = 71.5 Btu/lbm
*
(s1-s1) = 0.034 166 × 2.15 = 0.0735 Btu/lbm R
88.6
550.7
* *
(s2-s1) = 0.3974 ln 779.7 - 0.034 166 ln 805 = -0.0628 Btu/lbm R
*
*
(s2-s2) = (s2-sF2) - x2sFG2 = 0.034 166 × 6.12 - x2× 0.034 166(6.12 - 0.29)
= 0.2090 - x2× 0.1992
(s2-s1) = 0 = -0.2090 + x2 × 0.1992-0.0628 + 0.0735
*
=> x2 = 0.9955
*
(h2-h1) = CP0(T2-T1) = 0.3974(550.7-779.7) = -91.0 Btu/lbm
From D.2,
*
*
(h2-h2) = (h2-hF2) - x2hFG2 = 0.034 166×734.7[4.69 - 0.9955(4.69 - 0.32)]
= 117.7 − 0.9955 × 109.7 = 8.5 Btu/lbm
Turbine:
Pump
wT = (h1-h2) = -71.5 + 91.0 + 8.5 = 28.0 Btu/lbm
vF3 =
ZF3RT3
P3
=
0.0275 × 26.58 × 550.7
= 0.031 55 ft3/lbm
88.6 × 144
4
144
⌠ vdP ≈ vF3(P4 -P3) = -0.031 55(805-88.6) ×
wP = - ⌡
778 = -4.2 Btu/lbm
3
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.177E
A line with a steady supply of octane, C8H18, is at 750 F, 440 lbf/in.2. What is
your best estimate for the availability in an steady flow setup where changes in
potential and kinetic energies may be neglected?
Availability of Octane at
Ti = 750 F, Pi = 440 lbf/in.2
R = 13.53 ft lbf/lbm-R = 0.017 39 Btu/lbm-R
440
1209.7
Pri = 361 = 1.219, Tri = 1023.8 = 1.182
From D.2 and D.3:
*
(h1-h1) = 0.017 39 × 1023.8 × 1.15 = 20.5 Btu/lbm
*
(s1-s1) = 0.017 39 × 0.71 = 0.0123 Btu/lbm R
Exit state in equilibrium with the surroundings
Assume T0 = 77 F, P0 = 14.7 lbf/in.2
536.7
14.7
Tr0 = 1023.8 = 0.524 , Pr0 = 361 = 0.041
From D.2 and D.3:
*
(h0-h0) = RTC × 5.41 = 96.3
*
and
*
(s0-s0) = R × 10.38 = 0.1805
*
(hi -h0) = 0.409(1209.7 - 536.7) = 275.3 Btu/lbm
1209.7
440
* *
(si -s0) = 0.409 ln 536.7 - 0.017 39 ln 14.7 = 0.2733 Btu/lbm R
(hi-h0) = -20.5 + 275.3 + 96.3 = 351.1 Btu/lbm
(si-s0) = -0.0123 + 0.2733 + 0.1805 = 0.4415 Btu/lbm R
ψi = wrev = (hi-h0) - T0(si-s0) = 351.1 - 536.7 (0.4415) = 114.1 Btu/lbm
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.178E
A distributor of bottled propane, C3H8, needs to bring propane from 630 R, 14.7
lbf/in.2 to saturated liquid at 520 R in a steady flow process. If this should be
accomplished in a reversible setup given the surroundings at 540 R, find the ratio
of the volume flow rates Vin /Vout, the heat transfer and the work involved in the
process.
.
.
R = 35.04/778 = 0.045 04 Btu/lbm R
630
14.7
Tri = 665.6 = 0.947 Pri = 616 = 0.024
From D.1, D.2 and D.3 :
Zi = 0.99
*
(hi -hi) = 0.045 04 × 665.6 × 0.03 = 0.9 Btu/lbm
*
(si -si) = 0.045 04 × 0.02 = 0.0009 Btu/lbm R
Tre = 520/665.6 = 0.781,
From D.1, D.2 and D.3 :
Pre = = 0.21 , Pe = 0.21 × 616 = 129 lbf/in.2 ,
Ze = 0.035
*
(he-he) = 0.045 04 × 665.6 × 4.58 = 137.3 Btu/lbm
*
(se-se) = 0.045 04 × 5.72 = 0.2576 Btu/lbm R
*
*
(he-hi ) = 0.407 (520 - 630) = -44.8 Btu/lbm
520
132
* *
(se-si ) = 0.407 ln 630 - 0.045 04 ln 14.7 = -0.1770 Btu/lbm R
(he-hi) = -137.3 - 44.8 + 0.9 = -181.2 Btu/lbm
(se-si) = -0.2576 - 0.1759 + 0.0009 = -0.4326 Btu/lbm R
.
Vin
.
Vout
ZiTi/Pi 0.99 630 129
= Z T /P = 0.035 × 520 × 14.7 = 300.7
e e
e
wrev = (hi - he) - T0(si - se) = 181.2 - 540(0.4326) = -52.4 Btu/lbm
qrev = (he - hi) + wrev = -181.2 - 52.4 = -233.6 Btu/lbm
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
Mixtures
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.179E
A 4 lbm mixture of 50% argon and 50% nitrogen by mole is in a tank at 300 psia,
320 R. How large is the volume using a model of (a) ideal gas and (b) Kays
rule with generalized compressibility charts.
a) Ideal gas mixture
Eq.11.5:
Mmix = ∑ yi Mi = 0.5 × 39.948 + 0.5 × 28.013 = 33.981
R = 1545.36 / 33.981 = 45.477 lbf-ft/lbm-R
mRT 4 × 45.477 × 320
lbf-ft
V= P =
= 1.347 ft3
300 × 144
psi × in2/ft2
b) Kay’s rule Eq.12.84
Pc mix = 0.5 × 706 + 0.5 × 492 = 599 psia
Tc mix = 0.5 × 271.4 + 0.5 × 227.2 = 249.3 R
300
320
Reduced properties:
Pr = 599 = 0.50, Tr = 249.3 = 1.284
Fig. D.1: Z = 0.92
−
mRT
V = Z M P = Z VID gas = 0.92 × 1.347 = 1.24 ft3
mix
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.180E
R-410A is a 1:1 mass ratio mixture of R-32 and R-125. Find the specific volume
at 80 F, 200 psia using Kays rule and the generalized charts and compare to Table
F.9
Kay’s rule Eq.12.84
Pc mix = 0.5 × 838 + 0.5 × 525 = 681.5 psia
Tc mix = 0.5 × 632.3 + 0.5 × 610.6 = 621.45 R
200
539.67
Reduced properties: Pr = 681.5 = 0.293, Tr = 621.45 = 0.868
Table F.1: R = 1545.36 / 72.585 = 21.29 lbf-ft/lbm-R
Fig. D.1: Z = 0.82
ZRT 0.82 × 21.29 × 539.67 lbf-ft/lbm
= 0.327 ft3/lbm
v= P =
200 × 144
psi × in2/ft2
Table F.9:
v = 0.3174 ft3/lbm
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.181E
The R-410A in Problem 12.180E flows through a heat exchanger and exits at 280
F, 200 psia. Find the specific heat transfer using Kay’s rule and the generalized
charts and compare this to solution found using Table F.9.
Kay’s rule Eq.12.84
Pc mix = 0.5 × 838 + 0.5 × 525 = 681.5 psia
Tc mix = 0.5 × 632.3 + 0.5 × 610.6 = 621.45 R
Table F.1:
R = 1545.36 / 72.585 = 21.29 lbf-ft/lbm-R = 0.02736 Btu/lbm-R
CP mix = ∑ ci CP i = 0.5 × 0.196 + 0.5 × 0.189 = 0.1925 Btu/lbm-R
200
Reduced properties 1: Pr1 = 681.5 = 0.293,
Fig. D.1:
539.67
621.45 = 0.868
(h*1 − h1) = 0.45 × RTc = 0.45 × 0.02736 × 621.45 = 7.65 Btu/lbm
Reduced properties 2:
Fig. D.1:
Tr1 =
Pr2 =
200
681.5 = 0.293,
739.67
Tr2 = 621.45 = 1.19
(h*2 − h2) = 0.25 × RTc = 0.25 × 0.02736 × 621.45 = 4.25 Btu/lbm
The energy equation gives
1q2 = (h2 - h1) =
(h2 − h*2) + (h*2 − h*1) + (h*1 − h1)
= -4.25 + 0.1925 (280 – 80) + 7.65
= 41.9 Btu/lbm mix
Table F.9.2:
q = h2 – h1 = 176.26 – 126.34 = 49.92 Btu/lbm
The main difference is in the value of specific heat, about 0.25 Btu/lbm-R at
the avg. T, whereas it is 0.1925 Btu/lbm-R at 77 F.
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
Review Problem
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
12.182E
A new compound is used in an ideal Rankine cycle where saturated vapor at 400
F enters the turbine and saturated liquid at 70 F exits the condenser. The only
properties known for this compound are a molecular mass of 80 lbm/lbmol, an
ideal gas heat capacity of Cpo = 0.20 Btu/lbm-R and Tc = 900 R, Pc = 750 psia.
Find the specific work input to the pump and the cycle thermal efficiency using
the generalized charts.
T1 = 400 F = 860 R
1
.
WT
Turbine
.
QH
Ht.
Exch
T3 = 70 F = 530 R
2
Cond
P
4
.
-W P
3
x1 = 1.0
x3 = 0.0
Properties known:
M = 80, CPO = 0.2 Btu/lbm R
TC = 900 R, PC = 750 lbf/in.2
860
530
Tr1 = 900 = 0.956, Tr3 = 900 = 0.589
From D.1: Pr1 = 0.76, P1 = 0.76 × 750 = 570 lbf/in.2 = P4
Pr3 = 0.025, P3 = 19 lbf/in.2 = P2,
vf3 = Zf3RT3/P3 =
Zf3 = 0.0045
0.0045 × 1545 × 530
= 0.0168 ft3/lbm
19 × 144 × 80
4
wP = - ⌠
⌡ vdP ≈ vf3(P4 -P3) = -0.0168 (570 - 19) ×
3
144
= -1.71 Btu/lbm
778
qH + h4 = h1 , but h3 = h4 + wP => qH = (h1 - h3) + wP
From D.2,
*
(h1 - h1) = (1.9859/80) × 900 × 1.34 = 30.0 Btu/lbm
*
(h3 - h3) = (1.9859/80) × 900 × 5.2 = 116.1 Btu/lbm
*
*
(h1 - h3) = CP0(T1-T3) = 0.2(400-70) = 66.0 Btu/lbm
(h1 - h3) = -30.0 + 66.0 + 116.1 = 152.1 Btu/lbm
qH = 152.1 + (-1.71) = 150.4 Btu/lbm
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
Borgnakke and Sonntag
*
*
*
*
Turbine, (s2 -s1) = 0 = -(s2 - s2) + (s2 - s1) + (s1 - s1)
From D.3,
*
(s1 - s1) = (1.9859/80) × 1.06 = 0.0263 Btu/lbm R
530
19
*
*
(s2 - s1) = 0.20 ln 860 - 0.024 82 ln 570 = -0.0124 Btu/lbm R
Substituting,
*
*
(s2-s2) = - 0.0124 + 0.0263 = 0.0139 = (s2-sf2) - x2sfg2
0.0139 = 0.024 82 × 8.77 - x2 × 0.024 82 (8.77 - 0.075)
=> x2 = 0.9444
*
*
(h2-h2) = (h2-hf2) - x2hfg2
From D.2,
hfg2 = 0.024 82 × 900 (5.2 − 0.07) = 114.6 Btu/lbm
*
(h2-h2) = 116.1 - 0.9444 × 114.6 = 7.9 Btu/lbm
wT = (h1-h2) = -30.0 + 66.0 + 7.9 = 43.9 Btu/lbm
ηTH =
wNET
qH
=
43.9 - 1.7
150.4 = 0.281
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for
testing or instructional purposes only to students enrolled in courses for which this textbook has been
adopted. Any other reproduction or translation of this work beyond that permitted by Sections 107 or 108
of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.