MEDICAL SURGICAL NURSING DEPARTMENT- FACULTY
of NURSING- BENI-SUEF NATIONAL UNIVERSITY
2023-2024
Head of Medical surgical Nursing Department
Dr/ Elsayed Sabek
Program coordinator of Faculty of nursing, Beni Seuf National
University
Prepared by:
Dr Walaa Eid Zaki ( Course coordinator)
Lecturer, Medical Surgical Nursing. Faculty of nursing, Beni Suef University
Dr Emad Abdel halim
Lecturer, critical care and emergency Nursing. Faculty of nursing, Beni Suef University
Dr Hamdya Ahmed Ali
Lecturer, Critical care and emergency Nursing. Faculty of nursing, Beni Suef University
Dr. Mona abdel aty
Lecturer, Critical care and emergency Nursing. Faculty of nursing, Beni Suef University
Assist.L/ Reda Quarany
Assistant Lecturer, Critical care and emergency Nursing. Faculty of nursing, Beni Suef university
Assist.L/ Walaa Nady
Assistant Lecturer, Critical care and emergency Nursing. Faculty of nursing, Beni Suef university
Assist.L/ Marwa Khaled
Assistant Lecturer, Medical Surgical Nursing. Faculty of nursing, Beni Suef university
Demo/Safia Kamel
Demonstrator, Medical Surgical Nursing. Faculty of nursing, Beni Suef university
Demo/Heba Ramdan
Demonstrator, Medical Surgical Nursing. Faculty of nursing, Beni Suef university
Demo/Sabreen Mahmoud
Demonstrator, Medical Surgical Nursing. Faculty of nursing, Beni Suef university
Demo/Fatma Ali
Demonstrator, Medical Surgical Nursing. Faculty of nursing, Beni Suef university
Demo/Abdelrahman Sayed
Demonstrator, Medical Surgical Nursing. Faculty of nursing, Beni Suef university
Demo/ Mahmoud hassan
Demonstrator, Medical Surgical Nursing. Faculty of nursing, Beni Suef university
2
List of Content
Content
Nasogastric tube insertion
Page
4
Nasogastric tube feeding
15
CVC insertion
29
CVP measurement
34
CVC sampling & Removal
42
ECG
48
Stress ECG
61
Holter
66
Hemodialysis care
68
Plasmapheresis
81
Portcath care
92
Extravasation care
107
References
116
3
Introduction
The nurse should be familiar with the anatomy and physiology of the
nose, pharynx, esophagus, and stomach when caring for clients with NG
tubes.
The nares are the exterior openings to the nasal cavity. Usually, one nare
is larger and more patent than the other. The nasal floor is parallel to the
roof of the mouth. The end of the nasal cavity is narrow and ends at the
juncture of several bones, including a portion of the cribriform plate,
which is a very thin bone that, if fractured, could provide a direct
portal into the brain. For this reason, NG tube placement in clients with
suspected head trauma may be contraindicated.
The pharynx is a mucous membrane lined tube that begins at the nasal
cavity and is divided into three major regions: the nasopharynx, the
oropharynx, and the laryngopharynx. The epiglottis is a cartilaginous flap
of connective tissue located at the entrance to the larynx. During
swallowing, the larynx moves upward, and the epiglottis closes over the
glottis to prevent aspiration of food and fluid into the trachea. The
esophagus starts at the upper esophageal sphincter and runs down through
the diaphragm past the lower esophageal sphincter to the stomach.
Gastric intubation via the nasal passage (i.e. nasogastric route) is a
common procedure that provides access to the stomach for diagnostic and
therapeutic purposes. A nasogastric (NG) tube is used for the procedure.
It is a long polyurethane or silicone tube that is passed through the nasal
4
passages via the esophagus into the stomach. The placement of an NG
tube can be uncomfortable for the patient if the patient is not adequately
prepared with anesthesia to the nasal passages and specific instructions on
how to cooperate with the operator during the procedure.
Polyurethane or silicone tubes are better for long-term use (> 4-8
weeks) because they are more flexible and less irritating to tissues.
These tubes should be changed in line with the manufacturer’s
guidelines (usually every 30-90 days).
Polyvinylchloride (PVC) tubes should be used for a short period of
time (3weeks) usually for gastric drainage, decompression, lavage or
diagnostic procedures.
Definition of NGT:
Is a flexible plastic tube inserted through a nostril, down the posterior oro or
nasopharynx, and into the stomach or the upper portion of the small
intestine. It is typically used for decompression of the stomach or for
administration of nutrition or medication to clients who are at risk for
aspiration or unable to tolerate oral intake.
Indications of NGT Insertion:
A-Diagnostic Purpose
– Evaluation of upper gastrointestinal (GI) bleeding (i.e. presence,
volume).
5
– Aspiration of gastric fluid content.
– Administration of radiographic contrast to the GI tract.
B-Therapeutic Purpose
– Gastric decompression, including maintenance of a decompressed
state after endotracheal intubation.
– Relief of symptoms and bowel rest in the setting of small-bowel
obstruction
– Dilute and remove a poison.
– Aspiration of gastric content from recent ingestion of toxic material
– Administration of medication
– Feeding in malnourished patients
– Administer fluids and other substances (e.g., activated charcoal,
radiological contrast agents) when oral administration is not viable.
Contraindications of NG tube insertion:
There are two types of contraindications for any procedure or
intervention and are referred to as absolute and relative. An absolute
contraindication means the procedure or intervention may produce a
life-threatening situation and should be avoided if possible. A relative
contraindication means caution should be used because the possibility
of an adverse event is possible; therefore, benefits must outweigh the
risks.
A- Absolute Contraindications
– Severe maxillo – facial disorders, surgery or trauma (eg cribriform
plate disruption), due to the possibility of inserting the tube
intracranially. In this instance, an orogastric tube may be inserted.
– Recent nasal, pharyngeal or esophageal surgery
– Nasal CPAP
– Patients with oropharyngeal tumor
– Laryngectomy
– Esophageal obstruction, such as a neoplasm or foreign object and
esophageal tumors.
B- Relative contraindications
– Esophageal trauma, especially if caustic substances were ingested.
– Coagulation abnormality or anticoagulation therapy may cause
bleeding to the tissue from tube placement.
– Esophageal varices or stricture
– Recent banding or cautery of esophageal varice
6
Types of NG tubes
1-The Levin tube, the most commonly
used NG tube for feeding and medication
administration, has a single lumen, is
typically 90–110 cm/35–43 in long, and
they have a smaller bore with a size
ranging from 8 to 12 French.
2-The Salem-sump, also called the gastric
sump or ventral tube, It has two lumens: the
smaller lumen (colored blue) is left open to
the atmosphere for ventilation and the sump
or larger lumen is used for suction or
instillation of oral agents. The two-lumen
design permits continuous suction because
the smaller lumen vents the tube to
atmospheric pressure to reduce the risk that
a vacuum will form within the stomach and
cause the NGT to adhere to the gastric
mucosa. Their bore size ranges from 6 to 18
French, with those most commonly inserted
being 14 to 16 French.
3-Dobhoff tube, is a special type of NG tube
that is small bore and flexible, so it is more
comfortable for the client than a standard NG
tube. The tube is inserted with the use of a
guide wire, called a stylet that is removed after
correct tube placement is confirmed. A
Dobhoff tube also has weight on the end to
allow gravity and peristalsis to help advance
the end of the tube past the pylorus, providing
an additional barrier to reduce aspiration risk of
nutrition or medications administered.
7
Rational
Procedure steps
Assessment
Assess patient medical record for:
Check the physician’s order for inserting a
nasogastric tube.
Patient name and age.
Determine the purpose for the nasogastric tube.
Diagnosis of patient.
Medication of patient.
History of previous esophageal and nasal surgery.
Lab investigation e.g PT, PC and INR.
Assess patient for:
Presence of gag reflex by tongue depressor.
Mental state or ability to cooperate with procedure.
Preparation
-Checking the order clarifies
the procedure and type of
equipment required.
-To avoid mistake and
determine size of NG tube
according to patient age.
-Facilitates
outcomes.
evaluation
of
-To save time and effort and
Prepare Equipment:
– Nasogastric tube of appropriate size.
to ensure efficiency of the
tube.
Adult: 16-18F {1 French unit = 0.33 mm}
– Water-soluble lubricant
-Tongue blade
– Flash light
-Stethoscope
– Non-allergic tape
– Glass of water with ice chips (when person is
conscious) .
– Facial tissues
– Suction machine
– Bath towel or disposable pad
– Emesis basin
-Clamp
– Drainage bag
– Disposable gloves and PPE
– Normal saline (for irrigation only)
– Tommy syringe (irrigating syringe)
– PH indicator strip
-Oral hygiene
supplies
8
Prepare Nurse.
– Perform hand hygiene
– Put on disposable gloves and PPE (if needed).
-Reduce transmission of
microorganisms.
Prepare patient.
-Ensures that the procedure
-Check the patient’s identification. (Check client’s will performed on the correct
patient.
armband)
- An explanation reduces
-Explain the procedure, benefits, risks, complications, apprehension and facilitates
and alternatives to the patient or the patient's cooperation.
representative.
-To enable the patient to stop
the procedure if they wish.
-Baseline data concerning the
-Agree with the patient a signal by which he or she presence of bowel sounds and
can indicate to stop the procedure e.g. by raising a the amount of distention will
serve for future comparisons.
hand.
-obtain verbal consent from patient or relatives.
-Assess the patient’s abdomen.
-Because tearing may occur
when the tube passes into
nasopharynx.
Removing
dentures help prevent choking,
-Ask patient to remove eye glasses and dentures if as it may result from losing or
displacement of dentures.
present.
-Attempts to introduce a tube
into an obstructed naris may
cause
discomfort
and
unnecessary trauma.
-Check that the nostrils are patent by:
a- For conscious: Asking the patient to sniff with one
nostril closed. Repeat with the other side. For
unconscious: Nasopharyngeal suction if needed.
b- Examine naris with pen light for obstruction of
deformities as deviated septum or nasal polyps”
Prepare environment.
-Close curtains around the bed and close the door to
-Demonstrates respect for
the room, if possible.
dignity.
-Maintain bed at high comfortable position at waist
level to ensure good body mechanics.
9
Implementation
-Place a suction machine at the bedside if the patient -Provides a method for
is unresponsive or has difficulty swallowing.
clearing the patient’s airway
of vomitus.
-Assist the patient to high fowler’s position, at least a - An upright position is more
45° angle or higher. Place comatose clients in semi- natural for swallowing and
Fowler’s position. Note: The head should not be tilted protects against aspiration.
backwards or forwards..
- Monitor pulse oximeter and capnograph before, Provides baseline for objective
during and after procedure.
assessment of respiratory
If patient has increase in end-tidal carbon dioxide or status during tube insertion.
decrease in oxygen saturation, tube should not be
inserted until you determine patient stability.
-Drape patient chest with a bath towel or disposable - The patient might vomit as
pad and have an emesis basin at bed side patients bed. passage of the tube may
stimulate gagging.
-Using a small piece of tape, mark the distance which -To identify the length of tube
the tube is to be passed by measuring the distance on that needs to be inserted to
the tube from the patient’s bridge of the nose to ear ensure it is in the correct
lobe from the ear lobe to the bottom of the position.
xiphisternum (NEX measurement).
-Using water-soluble lubricant and lubricate about 2- -If aspirated; water-soluble
3inches (5-7.5 cm) of the tube with a thin coat of lubricant does not cause
lubricating jell that has been placed on gauze swab.
pneumonia, and lubricant
prevents damage to mucosa
during insertion.
.
Pinch ending of NGT during insertion (If patient -Iced tube becomes stiff and
vomitus Open tube on draindge bag or emesis basen). inflexible and causing trauma
Note: Do not ice tubes.
to nasal mucosa.
10
-Hold tube about 10cm from the tip and insert it into
nostril gently guiding it straight back and downward
along floor of nose with a twisting movement of tube
in fingers until resistance is met.
Note: If an obstruction is felt, withdraw the tube and
try again in a slightly different direction or use the
other nostril.
-At this point, ask the patient to start swallowing. An
assistant may be required to help give the fluids if the
patient is unable to take it.
Note: if the patient is comatose, absent gag reflex or
un -cooperate, do not attempt to use water orally to
pass tube. Neck positioning may facilitate passage
(flex head toward chest).
-Continue to advance tube gently each time patient
swallows until you reach point on tube previously
marked with tape.
- Helps tube follow path of
nasal passage.
-Check for position of tube in back of throat with
penlight and tongue blade.
-Tube may be coiled, kinked,
or inserted into trachea.
-If patient is gasping, cyanotic, or unable to speak,
stop procedure, remove the tube immediately, let
patient relax, re-lubricate tube, then reinsert.
-Secure tube to patient’s nose with tape. Make sure
the skin on the person’s nose is clean and dry. Cut a 3
to 4 inch strip of tape and cut it up the center 1 to 1.5
or 2 inches. Place the solid portion on the nose and
wrap the ends tightly around the tube.
-Tube may have entered
trachea instead of esophagus.
-To avoid unnecessary trauma
to the nose and nasopharynx.
-The swallowing action places
the epiglottis over the trachea
allowing the tube to enter the
esophagus.
-To ensure that tube reach the
stomach.
-Taping the tube to the nose
helps to keep it from slipping
out or being pulled out. Dry,
clean skin facilitates adhesion
of the tape to the nose.
- Check placement of tube in stomach by using the following methods:
1. Traditional method: (Not reliable alone but
Consider that:
more available)
- If air is difficult to hear,
Instill 20-30 mL of air into the tube with
the tube may not be in the
11
a large syringe while listening for the air
stomach.
bolus over the epigastric region.
- If the injected air is audible
in the mouth area, the tube
tip may have curled in the
upper Gl tract.
- If unable to instill air, the
tube may be kinked.
(AACN, 8th ed , 2024)
Indicator
strips
can
2. Advanced methods: ( more reliable and
distinguish between gastric
accurate)
acid and bronchial secretion
(pH<6)
A- Use syringe to gently aspirate stomach contents
-Do not inject an air bolus, as
and testing 2 ml of stomach contents with pH
the best practice is to test the
indicator strips. A pH of between 1 and 5.5 is
pH of the aspirated contents to
reliable confirmation that the tube is not in the
ensure that the contents are
lung.
acidic.
B- Chest or upper abdomen X-ray to confirm
position.
-According to purpose of insertion: Either attach tube
to suction or drainage bag or clamp or plug it, begins
lavage/ irrigation, instill medications, or begin gastric
gavage.
-Connecting the tube depends
on the purpose for which the
tube was inserted. Also, If
tube is left open, gastric
content may drain into gown
or linens.
Post care
Patient:
-Clean the tube of any remaining lubricant and
provide oral hygiene at least twice daily.
-Oral hygiene and cleansing
promote the patient’s comfort,
sense of wellbeing and
remove microorganism.
-Keep the patient comfortable “with a minimum head
- Position the patient with a
elevation of 30 degree.
minimum head elevation of 30
degree, to prevent gastric
reflux.
12
Equipment.
- Dispose of rubbish appropriately; rinse the
- Rinsing promotes cleanness.
equipment if it will be reused.
Nurse
- Hand washing deters the
spread of microorganisms.
-Remove gloves and PPE& hand washing.
Environment
-rearrange of environment.
Documentation
-Document the following:
Date and time of procedure
Size and type of tube inserted
Length of tube extending from nostril
How tube position confirmed.
Document and report any abnormalities as
needed
Measure and record the volume of drainage (if
any) at least every 8 hours.
Signature
Documentation provides
coordination of care.
Communicates to the other
members of the health care
team and contributes to the
legal record by documenting
the care given to the patient.
Also, provides data for
evaluating fluid balance.
Routine Nursing Care for Patient with NG Tube
Patient with NG tubes are at constant risk for developing adverse effects.
While caring for patient with NG tube, nurses monitor risks and adopt
strategies for patient safety and quality of care. The nurse should perform
the following interventions:
o
Keep the head of the bed 30 degrees or higher.
Patients with NG tubes are at risk for aspiration, especially if they are
receiving enteral nutrition. The head of the bed should always be raised
30 degrees or higher to prevent aspiration.
13
o
Prevent migration and/or dislodgement of the tube.
The NG tube should be fastened to the patient using a securement device
and taped/pinned to the patient’s gown to prevent the tube from slipping
from out of the stomach, migrating into the lungs, or being accidentally
removed.
– Daily assessment of patency of the tube, location, accuracy.
– Inspection of the nares for irritation. If so remove it and replace in the
opposite nares, daily cleansing using swab moistened with water
around the entrance of the tube is recommended to remove secretions.
– Inspect the adhesive tape for irritation of the underlying skin.
– Frequent oral hygiene. Apply cream for lips if they were cracked.
– Accurate record of intake and output, daily weighting.
– Minimize discomfort:
a. Generous lubrication, the use of topical anesthetic, and a gentle
technique may reduce the patient’s level of discomfort.
b. Throat irritation may be reduced with administration of
anesthetic lozenges (eg, benzocaine lozenges [Cepacol]) prior
to the procedure.
– Epistaxis may be prevented by generously lubricating the tube tip and
using a gentle technique.
o
Monitor for potential complications.
Signs of tube dislodgement into the respiratory tract include coughing,
shortness of breath, adventitious lung sounds, or decreasing oxygen
saturation levels.
Signs of esophageal perforation include neck or chest pain, dysphagia,
dyspnea, subcutaneous emphysema, or hematemesis.
Common complications
A- Minor Complications
- Nasal irritation.
- Epistaxis.
- Sinusitis.
14
B- Severe Complications
–Inadvertent placement in the trachea (Pulmonary intubation) leading
to pleural injury, pneumothorax, tracheobronchial aspiration,
pneumonia, and death if fluids or other agents are infused.
–Esophageal perforation.
–Inadvertent intracranial placement through a fractured cribriform
plate.
– Trauma to the nares or larynx, esophagus, and/or stomach during
insertion.
– Trauma or erosion of gastric mucosa, which is more common if
gastric suctioning is prolonged.
Definition of NGT Feeding:
Gavage is artificial method of giving persons fluids and nutrients through
a tube inserted to the stomach when oral intake is inadequate or
impossible.
Purposes:
A nasogastric gavage feeding is administered to:
o Provide total or supplemental nutrition.
o Restore fluid, electrolyte, and acid-base balance.
Indications of Gastric Gavage
Inability to ingest adequate nutrient orally e.g certain neurologic
disorders (stroke)
Hyper metabolic states (burns, multiple trauma, sepsis and cancer).
Following certain types of surgery (head and neck, esophaus)
Decrease level of consciousness (coma).
Malnutrition.
Endotracheal intubation.
Contraindication of Gastric Gavage
Absent bowel sounds
Advantages of Tube Feeding:
15
Tube
feedings
have
several
advantages
1-They are low in cost and safe
2-usually well tolerated by the patient
3- easy to use both in extended care facilities and in the patient’s
home.
4-May be continued for weeks without any danger to the patient
Remember the abbreviation NG TUBE before feeding
Complications of Tube Feeding:A-Gastrointestinal Complications:
*Diarrhea
*Nausea and Vomiting
B-Mechanical Complications:
*Aspiration Pneumonia
*Tube displacement or obstruction
*Nasopharyngeal irritation
C-Metabolic Complications:
*Hyper/hypoglycemia .
*hypokalemia (low potassium).
*Dehydration or over hydration
D-Others
*Constipation
*Cramping and Distention
Sinus infection
Nose bleeding
16
Rational
Procedure steps
Assessment
Assess patient medical record for:
Physician order.
Time, amount and type of ordered formula.
Medications to be administered before, during
and after feeding.
Type, amount of last feeding.
Assess patient for:
Assess patient’s height, weight, hydration
status, electrolyte balance, caloric needs and
intake and output (I&O)
Assess abdomen by inspecting for presence of
distention, auscultating for bowel sounds.
If patient connected with ETT, maintain cuff
inflated
Assess Formula for
Expiration date of feeding formula.
Be sure at room temperature
-Reading the written order
ensures that the procedure will
comply with the directives of the
physician.
- Checking ensures that correct
feeding will be administered and
outdated formula may be
contaminated.
-Provides baseline information to
measure nutritional improvement
after enteral feedings.
-To assess GIT motility.
Preparation
Prepare equipment:
– Formula.
– Tommy syringe, feeding bag.
– Clamp & rubber band.
– Disposable pad or towel.
– Water.
– Clean Gloves.
– Enteral feeding pump-if ordered.
-To save time and effort
– Stethoscope.
Prepare myself.
– Perform hand hygiene and put on disposable
gloves.
Prepare patient.
– Identify the client.
– Explain the procedure and its purpose.
-Reduce transmission of
microorganisms.
- Ensures that the procedure will
be performed on the correct
patient.
17
-Position the patient with head
elevated at least 30 to45 degrees or
normal position for eating as possible.
N.B, If patient is forced to lay supine,
reverse Trendelenburg position.
-To gain patient cooperation and
of bed reduce anxiety.
as near
- Gravity promotes the
distribution of food to the lower
place in level of the stomach & minimizes
the risk of aspiration.
-Place protective drape over patient chest.
-To prevent patient clothes and
linen from being wet.
Prepare environment.
-Close curtains around the bed and close the door
-Demonstrates respect for dignity.
to the room, if possible.
-Maintain bed at high comfortable position at
waist level to ensure good body mechanics.
Implementation
-Check the placement of gastric tube through
using pH indicator strip to test the pH (pH should
be 1-5.5).
To prevent accidental instillation
into the respiratory tract if the
tube has become dislodged.
- Aspirate stomach contents to determine amount
of residual and calculate the amount aspirated in
relation to the amount of previous last feeding, if
it is 50% or more from the last amount, return it
again to the stomach and wait for one hour and
repeat aspiration again, if the same amount
aspirated report to the doctor.
- Pinch the tubing.
-To prevent electrolyte imbalance.
- Gastric aspiration content
returning again because it is
mixed with gastric enzymes and
secretion essential for digestion.
-When using Toomey syringe:
-To promote infusion of formula
along tubing.
-Remove plunger from barrel of syringe and
attach barrel to nasogastric tube.
- Elevate syringe to 18 inches above the patient's
head.
- Fill syringe with formula and allow syringe to
empty gradually. Refilling until prescribed
18
- This procedure prevents air from
entering stomach.
-Graduals emptying of tube
feeding by gravity from syringe
or gavage bag and reduce risk of
diarrhea.
amount has been delivered to the patient.
When using feeding bag:
- Attach bag to the end of the feeding tube and
raise bag 18 inches above patient's head, fill bag
with prescribed amount of formula and allow bag
to empty gradually.
-Follow tube feeding with water in amount
ordered then clamp the proximal end of the tube
feeding.
-Provides patient with source of
water to help maintain fluid and
electrolyte balance.
-Label feeding bag with date and time, change the
bag and set every 12 to 24 hours.
-Observe patient response during and after tube
feeding.
Pain may indicate stomach
distension which may lead to
vomiting.
Post care
Patient:
- Keep the head of the bed elevated for at least 30 - To prevent gastric reflux and
aspiration.
to 60 minutes after a feeding.
- Provide oral hygiene at least twice daily.
-Remove microorganisms and
promotes comfort and hygiene of
the patient.
Equipment.
- Dispose of rubbish appropriately; rinse the
equipment if it will be reused.
- Rinsing promotes cleanness and
prepares the equipment for the
next feeding.
Nurse
-Remove gloves and PPE& hand washing.
- Hand washing deters the spread
of microorganisms.
Environment
-rearrange of environment.
19
Documentation
Date, time & amount of feeding formula.
Amount of water used to flush the feeding
A written summary facilitates the
documentation
of the procedure & provides a
record of comprehensive care.
tube.
Amount of gastric residual.
Any drugs instilled through the tube.
Unexpected outcomes.
Nurse signature.
Nursing Considerations for patients with NGT feeding:
1-If the patient has endotracheal tube; the cuff inflation should be
confirmed before feeding to avoid aspiration.
2-Monitor blood glucose level frequently as high carbohydrate
concentration of formula may exceed endogenous insulin supply and
cause hyperglycemia.
3-Weight the patient daily and compare with base line weight to avoid
weight loss or gain.
4-Auscultate the patient’s bowel sound frequently.
Definitions:
Gastrointestinal decompression: Is a process of reliving pressure by
removing accumulated gas and secretions from gastrointestinal tract
through nasogastric tube.
Gastric lavage:
Also commonly called stomach pumping or gastric irrigation, is the
process of cleaning out the contents of the stomach.
Indications:
1. Determine patency of NG tube.
2. Maintain patency of NG tube.
20
3. Wash stomach of toxic substances in poisoning treatment.
4. They may also be used in diagnosis and stop gastric hemorrhage.
5. To clean stomach before diagnostic procedures and to empty the
stomach after endoscopic procedure.
Contraindication:
Lavage is contraindicated when patients have a compromised,
unprotected
airway
and
in
patients
at
risk
of
gastrointestinal hemorrhage or perforation. Relative contraindications
include when the poisoning is due to a corrosive substance (strong acids).
Steps
Rational
Assessment
Assess patient medical record for:
Physician order.
Indication of gastric lavage.
Medications to be administered with
lavage fluid.
Reading the written order ensures that
Type, amount and temperature of gastric
the procedure will comply with the
lavage.
directives of the physician.
Need for sample of gastric aspirate.
Assess patient for:
Vital signs
LOC
Preparation
Prepare equipment:
– Container for irrigation solution
– Nasogastric tube connected to continuous or
intermittent suction
– Irrigating syringe
– Protective drape
– Urinary bag
To save time and effort.
– Clean gloves
– Irrigation solution ''saline or water''
–Stethoscope
– Graduated container
–Container for gastric content sample
21
Prepare myself.
– Perform hand hygiene and put on
disposable gloves and PPE (if needed).
-Reduce transmission of
microorganisms.
Prepare patient.
– Identify the client.
- Ensures that the procedure will be
performed to the correct patient.
– Explain the procedure and its purpose.
-To gain patient cooperation and reduce
anxiety.
– Assess abdomen by inspecting for
presence of distention, auscultating for
bowel sounds.
- Gravity promotes the distribution of
fluid to the lower level of the stomach &
minimizes the risk of aspiration.
-To prevent patient clothes and linen
from being wet.
– Assist the patient to a semi-fowler’s
position unless this is contraindicated.
– Place protective drape over patient chest.
Prepare environment.
-Close curtains around the bed and close the
Demonstrates respect for dignity.
door to the room, if possible.
-Maintain bed at high comfortable position
at waist level to ensure good body
mechanics.
Implementation
-If patient connected with continuous.
Clamp the suction tubing near the
-Clamping protects the patient from
leakage of gastric drainage.
connection site. Disconnect the
nasogastric tube from the suction unit and
place it over drape.
-Check the placement of gastric tube
through using pH indicator strip to test
the pH (pH should be 5.5 or less).
-To prevent accidental instillation into the
respiratory tract if the tube has become
dislodged.
22
-Pour irrigating solution into a container.
-Measuring the amount ensures that the
precise amount is delivered through the
tube. Using saline compensates for
electrolytes lost through nasogastric
drainage.
- Pinch the tubing.
- This procedure prevents air from
entering stomach.
-Remove plunger from barrel of syringe -To promote infusion of formula along
and attach barrel to nasogastric tube.
tubing.
- Elevate syringe to 18 inches above the
patient's head.
-Graduals emptying of tube feeding by
gravity from syringe or gavage bag and
reduce risk of diarrhea.
- Fill syringe with formula and allow
syringe to empty gradually. Refilling
until prescribed amount has been
delivered to the patient.
- If unable to irrigate. reposition the
patient and attempt irrigation again
after check with physician
- Reconnect tube with suction or
drainage bag according to prescription
- Measure and record the amount and
description of the irrigation and
returned solution.
Post care
Patient.
-return patient to comfort position.
Equipment.
- Dispose of rubbish appropriately;
-To maintain patient comfort.
- Rinsing promotes cleanness and prepares
the equipment for the next irrigation.
rinse the equipment if it will be
reused.
23
Nurse
-Remove gloves and PPE (if used) &
- Hand washing deters the spread of
microorganisms.
hand washing.
Environment
-Rearrange environment.
Documentation
Record & report :
Date, time & reason of gastric lavage.
Type
and
amount
solution.
of
-A written summary facilitates the
irrigating documentation of the procedure &
provides a record of comprehensive care.
Color, amount and consistency of
drainage.
Any drugs instilled through the tube.
Unexpected outcomes.
Nurse signature
Removal of Nasogastric tube
When the NG tube is no longer necessary for treatment, the primary care
provider will order the tube to be removed. The NG tube is removed as
carefully as it was inserted to provide as much comfort as possible for
the patient and to prevent complications.
Procedure of NGT Removal:`
24
Assessment
Reading the written order and ensure
that the procedure will comply with
the directives of the physician.
Assess patient medical record for:
Physician order.
Assess patient for:
Absence of indications of tube insertion.
Vital signs.
Preparation
Prepare equipment:
• Tissues
• 50-mL syringe
• Non-sterile gloves
• Additional PPE, as indicated
• Stethoscope
• Disposable plastic bag
• Bath towel or disposable pad
• Normal saline solution for irrigation
(optional)
• Emesis basin
To save time and effort.
Prepare myself.
– Perform hand hygiene and put on disposable
gloves and PPE (if needed).
-Reduce transmission of
microorganisms.
Prepare patient.
– Identify the client.
- Ensures that the procedure will be
performed on the correct patient.
– Explain the procedure and its purpose.
-To gain patient cooperation and
reduce anxiety.
– Assess abdomen by inspecting for presence
of distention, auscultating for bowel
sounds.
– Assist the patient to a semi-fowler’s position
unless this is contraindicated.
– Place protective drape over patient chest and
give paper tissues to patient.
25
- minimizes the risk of aspiration.
-To prevent patient clothes and linen
from being wet and avoid contact with
gastric secretion. To blow nose when
tube is removed
Prepare environment.
-Close curtains around the bed and close the
-Demonstrates respect for dignity.
door to the room, if possible.
-Maintain bed at high comfortable position at
waist level to ensure good body mechanics.
Implementation
-auscultate abdomen for presence of bowel
sound
-Verifies return of peristalsis
-Discontinue suction and separate tube from
suction and carefully remove adhesive tape
from patient's nose.
- allow for its unrestricted removal of
tube
- Attach syringe and flush with 10 mL normal
saline solution .
- Saline solution clears the tube of
secretions, feeding, or debris.
- Instruct patient to take a deep breath and hold -To prevent accidental aspiration of
it.
gastric secretions in tube. Careful
removal minimizes trauma and
discomfort for patient.
-Clamp tube with fingers by doubling tube on -Clamping prevents drainage of
itself. Quickly and carefully remove tube while gastric contents into the pharynx and
patient holds breath.
esophagus.
Post care
Patient
-return patient to comfort position.
- perform mouth care to patient and provide
paper tissues to blow nose.
-Continue to monitor patient for 2 to 4 hours
after tube removal for gastric distention,
nausea, or vomiting.
Equipment
26
-To maintain patient comfort.
- Place tube in disposable plastic bag.
Nurse
-Remove gloves& hand washing.
-Hand washing deters the spread of
microorganisms.
Environment
-Rearrange environment.
Documentation
Record & report :
Date, time
A written summary facilitates the
documentation of the procedure &
provides a record of comprehensive
care.
Unexpected outcomes
Patient tolerance
Nurse Signature
27
Central Venous Catheter (CVC) maneuver
Outlines:
Central venous catheter insertion:
Indication
Contraindication
Procedures for insertion
Complication
Central venous pressure monitoring:
Monitoring by water manometer
Monitoring by transducer
Blood sampling from CVC
Dressing change
Removing of CVC
28
CVC insertion
Introduction:Central venous catheters (CVCs) are a commonly used modality
throughout the medical center and especially in the intensive care units,
serving vital role in the management of critically ill patients.
Definition of CVC:Central venous catheter (CVC), also known as a central venous line
(CVL), or central venous access (CVA). It is a long, thin, flexible tube
placed into a large (central) vein in the neck, upper chest or groin. This
type of catheter has special benefits in that it can deliver fluids into a
larger vein for a longer period of time, usually several weeks or more.
29
Indication of CVC insertion:
Vascular Access:
Emergency venous access and failure of peripheral access.
As an alternative for repetitive venous cannulation of
chronically ill patients or patients with small thrombosed, or
difficult to find veins.
Long term IV access anticipated.
Provision of Medications or Solutions:
Drug infusions that could otherwise cause phlebitis or sclerosis
(e.g., vasopressors and hyperosmolar solutions)
- Central venous pressure (CVP) monitoring.
- Volume loading: Fluid resuscitation (including blood products).
Repeated Blood Sampling:
Although not an indication we usually consider, the ability to
draw blood samples from a central line will negate multiple
needle pokes for the patient who needs frequent blood
sampling.
Introduction of Pacemakers or Pulmonary Artery Catheters:
For urgent and short term hemodialysis.
Relative contraindications of CVC insertion:
1. Bleeding disorders or current anticoagulation or thrombolytic
therapy.
2. Distortion of local anatomy or landmarks.
3. Vasculitis, cellulitis, burn, severe dermatitis or other infection over
the anticipated insertion site.
4. Suspected acute or prior injury to the vein.
5. Pneumothorax or hemothorax.
6. Morbid or marked obesity.
7. Mastectomy on the side of insertion.
30
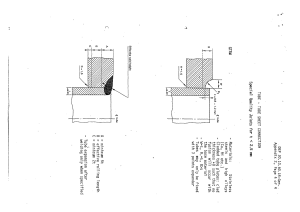
Sites of CVC insertion
Procedure of CVC Insertion:a- Equipment.
b- Procedure.
Post-Catheter Placement.
Equipment
Facilities to monitor the patient – particularly ECG
Central venous catheter kit.
Sterile gloves (appropriate size).
Sterile gown
proline suture (Size 3.0)
large dressing pack
2 x10ml syringes
2 x 5ml syringe
Local anesthetic (2% Lidocaine)
Antiseptic solution (0.5% Chlorhexidine in Alcohol 70%)
10ml 0.9% Sodium chloride for injection
18G and 23G needles
Rolled towel or sandbag.
31
o
o
o
o
o
o
o
o
o
o
o
o
Procedure of insertion:
Explain procedure to the patient.
Ensure ECG, BP and SaO2 are monitored.
The physician will do scrubbing and wear gown and glove.
Position the patient – supine with head slightly down. Turn the
head away from selected site if requested, place a rolled towel or
sandbag vertically between scapulae and provide gentle traction on
the arm (in the case of a subclavian approach).
The skin is prepared with 0.5% Chlorhexidine in Alcohol 70% and
draped.
Local anaesthetic is administered.
The central venous catheter is inserted into the superior vena cava
using a sterile Technique.
Blood is drawn back through the catheter and each lumen is
flushed with saline.
The catheter flange is sutured into position on the skin.
The site is cleaned and an occlusive dressing applied.
Check the position of the line on X-ray prior to the infusion of
fluids.
Ensure that nursing documentation is completed.
Post-Catheter Placement
Aspirate blood from each port
Secure catheter with sutures
Dispose all sharps.
32
Place an occlusive sterile dressing.
Flush lumens with saline to maintain patency.
Obtain a chest x-ray (for correct placement checking).
Monitor site for bleeding.
Assess vital signs and breathe sounds.
Document insertion, site, dressing and flushing.
Potential complications of CVC Insertion:
Hemorrhage:
Catheter occlusion: by a blood clot or kinked tube - regular
flushing of the CVC line and a well secured dressing should help to
avoid this.
Infection
Air embolus.
Catheter displacement
Others important Complications of CVC insertion
Venous thrombosis
Arterial puncture
Pneumothorax and hemothorax
Cardiac tamponade
Tracheal injury
Nerve injury
pulmonary embolism
Cardiac dysrhythmia
Special considerations: Arrange for daily chest X-rays as ordered to check catheter
placement. Change the dressing every 24 to 48 hrs or according to
agency policy.
After insertion, watch for signs and symptoms of pneumothorax,
such as shortness of breath, uneven chest movement, tachycardia,
33
and chest pain. Notify the practitioner immediately if such signs
and symptoms appear.
Use sterile technique when performing dressing, tubing, and
solution changes for a central venous access catheter. (See
“Changing the dressing on a central venous access catheter,”
Assess the site for signs and symptoms of infection, such as
discharge, inflammation, and tenderness.
To prevent an air embolism, close the catheter clamp, or have the
patient perform the Valsalva maneuver or exhale each time the
catheter hub is open to air.
Routinely evaluate catheter necessity and discontinue catheter use
as soon as it’s no longer needed to reduce the risk of vascular
catheter-associated infection.
Routinely flush the access device after blood sampling, before
and after medication administration, with discontinuation of an
infusion, and when the catheter isn’t in use.
CVP Measurment Central Venous Pressure (CVP)
Definition: It is the pressure of blood in the thoracic vena cava (right atrium) .It
reflects the amount of blood return to the heart and the ability of the heart
to pump the blood to the arterial system.
Normal CVP reading is in midaxillary line (2:6 mmhg or
5:10cmH2O)
Ideally, pressure should be measured from the distal (tip) lumen,
with no other infusions running through that lumen, but when distal
lumens are unavailable, differences from other lumens are usually
insignificant.
34
Purposes of CVP measurement
To assess patient’s fluid volume status
To assess preload of the heart
Provide information about the right ventricular function and right
side hemodynamics
Evaluation of patient response to therapy.
CVP Recording
Phlebostatic axis
CVP is usually recorded at the mid-axillary line where the manometer
arm or transducer is level with the phlebostatic axis. This is where the
fourth (4th) intercostal space and mid-axillary line cross each other
allowing the measurement to be as close to the right atrium as possible.
Methods of CVP monitoring:CVP is measured using an indwelling central venous catheter and a
pressure manometer or transducer.
CVP measurement using a manometer
Steps
Rationale
Assessment:
Patient's medical record:
a. Physician order
b. Diagnosis
c. Client's medications, and treatments
d. Last CVP reading
e. Last feeding time
b-To identify condition that
increase or decrease CVP
reading.
d-To obtain base line data
e-To prevent aspiration
35
Patient :
a- Cardiopulmonary status
a. Assess base line data
b-Assess factors that increase risk of aspiration as:
- Cuff inflation (for patient with ETT or TT)
- Ability to lie at supine position
- Level of consciousness
b. Deflated cuff increase risk of
aspiration
Machine if present as:
c- Mechanical ventilator for PEEP level (if patient connected with
MV)
d- Syringe Pump or Infusion Pump.
c. To identify factor that affect
CVP reading (High PEEP
increase intra thoracic pressure
that increase CVP reading)
d. All infusion must be
temporally stopped during
measurement except certain
infusion as (Intropics).
Preparation:
To save time and effort
A-For equipment : (Prepare necessary equipment)
IV solution (normal saline)
Two IV infusion set
IV pole
Water manometer
Disposable gloves.
Two different syringes.
Three way stopcock
B-For self
To minimize risk of infection.
1. Hand washing.
2. Put on disposable gloves
To maintain patient privacy.
C- For environment
-Close doors, windows and pull curtains.
D- For patient
To ensure correct patient.
To gain patient cooperation and
to decrease fear and anxiety.
1. Identify patient using two identifiers.
2. Explain procedure to the patient.
3. Implementation:
Connect three way stopcock to the distal lumen (if present) of
36
To prevent air embolism
CVC.
Connect infusion to IV line and expel air.
Connect IV solution to the three way of the catheter.
Open the three way between IV line solution and the patient.
Allow the normal saline to drip rapidly for few second.
For accurate measurement
To ensure patency of CVC
To confirm placement of CVC
Perform flush back.
Turn the three way stopcock off to the patient.
Attach the water manometer and open the three way between
infusion and manometer until air completely removed from
manometer then turn off.
Place patient in supine position unless contraindicated (if not
possible place patient in semi sitting position from 0 to 45
degree)
Locate the zero point in the patient chest (fourth intercostal space
at the mid-axillary line).
Turn off any additional lines and pumps (Unless contraindicated)
given via the same lumen that the manometer is attached to.
Open the three way between
water manometer and patient.
37
To avoid risk of air embolism.
To evacuate air from
manometer.
To ensure base line of the
manometer at the same level to
the right atrium
At the same level to the right
atrium
To ensure a closed system
between the manometer and the
right atrium
To allow fluid to drip from the
manometer to the right atrium
Watch for fluid falls at the
manometer until fluctuation
occurs and take measurement at
the end of expiration.
To minimize the effect of intrathoracic pressure
4. Post care:
For patient
Return the patient to the comfortable position.
Disconnect the water manometer and IV solution.
Reconnect infusions at preset rate (if stopped during
measurement).
To provide comfort and to
avoid respiratory distress
For equipment
Cover the ports of three way stopcock, water manometer and IV
set.
Discard disposable equipment.
For environment
Open doors, windows and remove curtains.
For self
Remove gloves and hand washing.
To minimize risk of infection.
5. Documentation:
Date and time.
Reading of CVP.
PEEP level(if patient
connected with MV)
Any complications that
appear during procedure.
Position of patient during procedures
Signature.
38
Measuring CVP using a transducer
Transducer system setup
39
Steps
Make sure that the patient is attached to a cardiac monitor with
pressure monitoring capability.
Make sure that the alarm limits are set appropriately for the patient’s
current condition and that the alarms are turned on, functioning
properly, and audible to staff.
If the patient has a CV catheter with multiple lumens, the distal port
is dedicated to continuous CVP monitoring.
Setup transducer system
The CVC will be attached to intravenous fluid within a pressure bag.
Ensure that the pressure bag is inflated up to 300mmHg. (Prevents
catheter from clotting by allowing 3-4 ml/hr flush solution to be
delivered through the catheter.
Label the tubing at the distal end (near the patient connection) and at
the proximal end (near the source container) to reduce the risk of
misconnection if multiple IV lines will be in use.
Place the patient flat in a supine position if possible. Alternatively,
measurements can be taken with the patient in a semi-recumbent
position. The position should remain the same for each
measurement taken to ensure an accurate
comparable result
Tape the transducer to the phlebostatic axis or as
near to the right atrium as possible. (Mark the
appropriate place on the patient’s chest so that
all
subsequent
measurements
use
the
same
location).
Turn the stopcock next to the transducer off to the
patient and open to air. Remove the cap to the stopcock
port.
40
Press the zero button on the monitor and wait
while calibration occurs.
When 'zeroed' is displayed on the monitor, replace
the cap on the three-way tap and turn on to the
patient. Place a new sterile, non-vented cap on
the stopcock to maintain sterility.
Perform a square waveform test (dynamic response
test) to verify the accuracy of the pressure
monitoring system.
Read the CVP value by measuring the mean of the
wave at end-expiration. The monitor will also
provide a value on the digital display(blue line in
this image)
(The waveform undulates as the right atrium contracts and
relaxes, emptying and filling with blood)
Return the bed to the lowest position to prevent falls and maintain
patient safety.
Remove and discard your gloves and other personal protective
equipment if worn.
Perform hand hygiene.
Document the procedure.
Factors that affect CVP:o Factors that increase CVP :
Cardiac tamponade
• Decreased cardiac output
Forced exhalation
• Heart failure
• Pleural effusion
Hypervolemia
Pulmonary Embolism
•Pulmonary Hypertension
41
Mechanical ventilation
•Tension pneumothorax
Application of positive end-expiratory pressure (PEEP).
B- Factors that decrease CVP :
Deep inhalation
Hypovolemia
Blood Sample aspiration from CVC
Definition:
It is the aspiration of blood sample from CVC by using the syringe. Blood
sampling the use of strict sterile no-touch technique is necessary to
reduce the risk of vascular catheter–associated bloodstream infection. In
addition, CVC blood sampling should not be performed using the same
catheter lumen that is used for drug infusions.
Only the minimal amount of discard blood should be obtained to
help prevent nosocomial anemia.
Equipment:
- Non sterile gloves
- Blood tubes and /or blood culture bottles for required tests.
- Alcohol swab
- Two syringes (10 ml)
- Specimen tubes.
- 10mls of sodium chloride 0.9% (Normal saline)
- Sterile gauze and dressing.
Steps:
1-Prepare equipment
2-Prepare myself (hands washing and wear non-sterile gloves).
3- Identify the patient and explain the procedure to the patient.
4-Stop any infusion running through CVC (don't stop emergency drugs).
5- Use the largest lumen (usually distal) of multi-lumen CVCs whenever
possible.
42
6- Close clamp between CVC and infusions.
7- Disconnect the tubing from the injection cap.
8- Disinfect the injection port with alcohol swab
9- Attach 10 ml syringe in to CVC catheter port
10- Open clamp on CVC and gently withdraw 10 ml blood from CVC
11- Clamp CVC and remove syringe.
12- Connect another 10 ml syringe and open the clamp between syringe
and CVC.
13- Aspirate desired amount of blood for lab investigation
14-Return blood aspirated firstly. (Check for blood clot before returning,
if present, discard it).
15- Attach syringe containing 0.9% sodium chloride to the
closed port of CVC.
16-Open the clamp and flush CVC with 0.9% sodium chloride.
17- Clamp CVC, reconnect the infusion.
18- Open the clamp between CVC and infusion
19- Fill blood tubes with appropriate quantity of blood
20- Label tube with patients name, unit, and type of the test.
Flushing and locking
Aspirating for a blood return and flushing a CVC is a routine step to
assess
catheter
patency
before
each
infusion,
prevent
mixing
incompatible medications and solutions after infusion, and prevent
catheter occlusion after blood sampling. Locking is performed after the
final flush to maintain patency and prevent occlusion in a device that’s
used intermittently. If the system is used intermittently, the flushing and
locking procedure will vary by facility preference, the medication
administration schedule, and the type and size of the catheter. Catheter
must be regularly flushed. To maintain patency in catheters used
intermittently, facilities may use a heparin flush solution available in
prefilled syringes of 10 units of heparin per milliliter or preservative-free
43
normal saline to lock the catheter. (According to patient condition and
facility policy).
Changing the dressing on a central venous access catheter
Despite the various designs and applications of central venous access
devices, certain aspects of dressing changes apply to all device types. The
sterility and integrity of the device must be maintained at all times to
reduce the risk of vascular catheter–associated infection. Transparent
semipermeable dressings should be changed every 5 to 7 days, and gauze
dressings should be changed every 2 days. If signs and symptoms of
infection are present, or if the dressing becomes visibly soiled, loosened,
or dislodged, change the dressing immediately to closely assess, clean,
and disinfect the site
Equipment for dressing and removal:
- Dressing pack
- Sterile scissors (if tip of CVC required for microbiology)
- Sterile container (if required for CVC tip)
- Stitch cutter and forceps
-Non sterile gloves
-Adhesive tape.
-Sterile gloves
- Disinfectant solution (Chlorhexidine)
44
Steps:
Determine the date of the last dressing change.
Perform hand hygiene.
Assemble the supplies on a sterile field.
Put on gloves to comply with standard precautions.
Remove the existing dressing by lifting the edge of the dressing at
the catheter hub and gently pulling the dressing perpendicular to
the skin toward the insertion site to prevent catheter dislodgment
and tearing or stripping of fragile skin.
If necessary, use a non-acetone adhesive remover to remove
adhesive from the patient’s skin, because a product containing
acetone can harm the catheter.
Discard the dressing in an appropriate receptacle.
If a chlorhexidine-impregnated sponge dressing was used to
provide sustained antimicrobial action at the insertion site, remove
and discard it in an appropriate receptacle.
Remove the engineered stabilization device and discard it in the
appropriate receptacle.
Assess the catheter–skin junction and surrounding skin for
bleeding, redness, swelling, tenderness, induration, and drainage.
Inspect the catheter for cracks, leakage, kinking or pinching, and
mechanical problems.
Remove and discard your gloves.
Perform hand hygiene.
Put on sterile gloves.
Measure the external catheter length using a sterile tape measure to
make sure that the catheter hasn’t migrated.
45
Clean the catheter insertion site with chlorhexidine to provide skin
antisepsis; if the patient is sensitive to chlorhexidine, use tincture
of iodine, povidone-iodine, or alcohol swabs.
Allow the area to air-dry completely. If povidone-iodine solution is
used, apply it using swabs. Begin at the catheter insertion site and
move outward in concentric circles.
Allow the solution to remain on the skin until it dries completely
(for at least 2 minutes).
Apply a skin barrier solution according to the manufacturer’s
instructions to reduce the risk of medical adhesive–related skin
injury. Don’t use compound tincture of benzoin, because it may
increase the bonding of adhesives to the skin, causing skin injury
when the adhesive-based engineered stabilization device is
removed.
Stabilize and secure the catheter, using an engineered stabilization
device,
if
available;
stabilization
device
an
is
engineered
recommended
because it reduces the risk of unintentional
catheter dislodgment
If
applicable,
place
a
chlorhexidine-
impregnated sponge dressing at the catheter base. To facilitate
future removal, position the chlorhexidine-impregnated sponge
dressing with the catheter resting on or near the radial slit of the
dressing. The edges of the slit must touch to maximize
antimicrobial action.
NURSING ALERT Use chlorhexidine-impregnated dressings with
caution in premature neonates and in patients with fragile skin or
complicated skin pathologies, because contact dermatitis and pressure
necrosis can occur
46
CVC removal
This is an aseptic technique performed by a doctor or qualified nurse.
Steps:
1-Hand washing
2-Explain procedure to patient
3- Position patient lying flat in bed with one pillow under shoulders and
head, it may prevent air being aspirated into the venous system
4-Put on non-sterile gloves
5-Stop any IV infusions running through CVC
6-Remove old dressing
7- Put on sterile gloves
8- Clean insertion site with cleansing solution (chlorhexidine)
9- Remove the sutures.
10- Instruct patient to take deep breath and perform Valsalva maneuver as
catheter is withdrawn in smooth, continuous motion. Valsalva maneuver
reduces the risk for air embolus by decreasing negative pressure in
respiratory
system.
11- As the catheter is withdrawn; apply gentle pressure around the exit
site using a sterile dressing. Stop removal procedure if resistance is met
while removing catheter.
12- If the tip of the CVC is required for microbiology, it should be placed
into a sterile container
13- Apply 2x2 gauze dressing over the site and secure tape.
14- Reposition the patient for comfort and observe the site closely for 30
minutes for any abnormalities.
47
Anatomy and physiology of the heart:
The heart is situated in the anterior thoracic cavity, just behind the
sternum and above the diaphragm; generally, the size of an individual’s
heart is about the same as that person’s clenched fist. In an adult, the
heart averages 12 cm in length and 8 to 9 cm in breadth at the broadest
part. The weight of the normal heart averages 330 g in men, and 245 g in
women. No significant differences exist in ventricular wall thickness
between men and women.
Layers of the Heart
The four distinct layers of the heart wall are:
(1) Pericardium: the outermost fibrous pericardium is a thick envelope that is
tough and inelastic, inside the fibrous layer is an inner, double-walled
membrane described as the pericardium. The two layers are labeled parietal
(outer layer) and visceral (inner layer, also known as the epicardium.
48
(2) The epicardium, also known as the visceral pericardium, is tightly adhered
to the heart and the base of the great vessels. The coronary arteries lie on the
top of the epicardium.
(3) Myocardium is a thick, muscular layer that includes all the atrial and
ventricular muscle fibers necessary for contraction.
(4) Endocardium : The innermost layer of the heart is the endocardium, which
is a thin layer of endothelium and connective tissue lining the inside of the
heart.
Cardiac Electrophysiology:
The cardiac conduction system generates and transmits electrical impulses that
stimulate contraction of the myocardium. Under normal circumstances, the
conduction system first stimulates contraction of the atria and then the ventricles.
The synchronization of the atrial and ventricular events allows the ventricles to fill
completely before ventricular ejection, thereby maximizing cardiac output.
Three physiologic characteristics of two types of specialized electrical cells, the
nodal cells and the Purkinje cells, provide this synchronization:
Automaticity: ability to initiate an electrical impulse
Excitability: ability to respond to an electrical impulse
Conductivity: ability to transmit an electrical impulse from one cell to
another
49
Conduction system:
To analyze electrical activity within the heart, it is helpful to understand
the main areas of impulse propagation and conduction:
Sinoatrial (SA) node.
The SA node is located at the junction of the superior vena cava and the
right atrium. The SA node is considered the natural pacemaker of the
heart because it has the highest degree of automaticity, producing the
fastest intrinsic heart rate (HR 60-100 b/m).
Internodal pathways.
The electrical impulses initiated by the SA node are conducted along the
myocardial cells of the atria via specialized tracts called internodal
pathways. The impulses cause electrical stimulation and subsequent
contraction of the atria
Atrioventricular (AV) node.
The AV node is located on the right side of the inter atrial septum on the
floor of the right atrium, electrical impulses initiated in the atria are
conducted to the ventricles only via the AV node Intrinsic rate is 40–60
bpm.
The AV node performs the following essential functions to support
cardiac conduction:
1. The AV node delays the conduction impulse from the atria (0.8 to 1.2
seconds).
2. The AV node controls the number of impulses that are transmitted from
the atria to the ventricles.
3. The AV node acts as a backup pacemaker if the faster SA node fails.
Bundle of His.
50
Located at the top of the inter ventricular septum, this bundle of fibers
extends directly from the AV node and connects the atria and ventricles
electrically.
Bundle branches.
The bundle of His splits into two conduction paths called the right and
left bundle branches. These bundles carry electrical impulses at high
speed to the tissue of the interventricular septum, and to each ventricle
simultaneously.
Purkinje system.
The bundle branches terminate with this network of fibers, which spread
electrical impulses rapidly throughout the ventricular walls. Intrinsic rate
is 20–40 bpm.
51
Electrocardiogram (ECG)
Introduction
Coronary heart disease remains a leading cause of mortality worldwide.
Promptly recognizing and treating acute coronary syndromes, such as STsegment elevation myocardial infarction (STEMI) and non-STEMI acute
coronary syndrome, can reduce and prevent cardiac arrest. One of the
most valuable and frequently used diagnostic tools, an electrocardiogram
(ECG) can display the heart’s electrical activity as waveforms. Impulses
moving through the heart’s conduction system create electrical currents
that can be monitored on the body’s surface
Definition of ECG
Is the graphic representation of the electrical currents of the heart. The
ECG is obtained by placing disposable electrodes in standard positions on
the skin of the chest wall and extremities.
A standard 12-lead ECG contains six limb lead images and six chest
(precordial) lead images, and the correct placement of these leads is
vitally important to avoid misdiagnosis
Indication of ECG:
Dysrhythmias such as (AF. SVT. VT. VF).
The effects of antiarrhythmic medications.
Conduction abnormalities and chamber enlargement.
Myocardial ischemia, injury or infarction.
Cardiac effects of electrolyte disturbances (high or low calcium and
potassium levels)
Check how well mechanical devices that are implanted in the heart,
such as pacemakers
52
Types of ECG procedure:
Standard 12 lead ECG (Resting ECG).
Stress or exercise ECG (treadmill test).
Ambulatory ECG (Holter monitoring)
Right ECG
Posterior ECG
Standard 12 lead ECG procedure
Steps
Rational
A) Assessment
1- Patient’s medical records:
Physician’s order
Patient’s name, age and gender.
Diagnosis of the patient
Patient’s history of cardiac dysrhythmias or
cardiac surgery (CABG).
Previous ECG.
Lab investigation (CK, CKMB and troponin,
serum potassium, calcium and magnesium).
2- Patient
Vital signs
Level of consciousness
Pacemaker
Patient’s extremities and chest area.
To identify types of ECG
Identify indication for
ECG
To compare recent ECG
with previous.
For areas of breakdown, or
excessive hair that might
with
electrode
Signs and symptoms as chest pain, interfere
placement.
tachycardia, palpation ,cyanosis, dizziness)
B) Preparation
1-Equipment :
ECG machine with recording paper
Electrodes or disposable pre-gelled
electrodes
Hair clippers (optional, depending on hair at
53
electrode sites)
4 × 4 gauze pads or washcloth
Marking pen
Bath blanket or sheet
Gloves, alcohol pad.
Additional PPE, as indicated.
Skin cleanser and water, if necessary
2-For ECG machine:
Place the ECG machine close to the patient’s
bed.
Check the cable and wires for fraying or
breakage, and replace them or obtain another
machine if necessary.
Plug the cord into the wall outlet, or ensure
proper functioning if the machine is battery
operated.
Turn on the machine and input the required
patient information.
Ensure that the paper speed selector is set to
The machine will record a
normal
standardization
the standard 25 mm/second and that the
mark—a square that’s the
machine is set to full voltage.
height of 2 large squares or 10
Calibrate to 10mm/mV.
small
Ensure that there is sufficient Paper.
recording paper
3-Environment :
Close doors, windows and pull curtains.
Ensure adequate lightening.
Ensure the environment is warm.
54
squares
on
the
Raise the bed to waist level when providing
patient care to prevent caregiver back strain.
4- For self:
Hand washing.
Wear gloves and PPE (if needed).
5-For Patient :
Confirm the patient’s identity using at least
two patient identifiers.
Explain the procedure to the patient.
Tell the patient that the test
records the heart’s electrical
activity, and that it may be
repeated at certain intervals.
Emphasize that no electrical
current will enter the body.
Also, tell the patient that the test
typically
takes
just
a
few
minutes
C)Implementation
Position the patient in a supine position in the To minimize muscle trembling,
which can cause electrical
center of the bed with arms at the sides.
interference.
Raise the head of the bed if necessary.
To promote comfort.
Make sure the feet do not touch the beds
footboard.
Ensure the patient’s arms and legs remain
relaxed
Expose the patient’s arms, legs, and chest,
55
and then cover the patient appropriately with
a bath blanket or sheet.
Select the electrode sites. Select flat, fleshy
areas to place the limb lead electrodes. Avoid
muscular and bony areas.
If the patient has an amputated limb, choose
a site on the residual limb.
If an area is excessively hairy, clip the hair
(don’t shave).
Wash the electrode site with soap and water Which helps remove the outer
and a washcloth and wipe them with a dry layer to facilitate electrical
washcloth or gauze pad to roughen the skin. signal transmission. If necessary
Mark the sites with an indelible marker if
serial ECGs are performed.
Connect the lead wires to the electrodes.
Note that the tip of each lead wire is lettered
and color-coded for easy identification
Red RT arm
Black RT leg
Yellow Lt arm
Green
Lt Leg
Apply disposable pre-gelled electrodes to the To guarantee the best connection
patient’s wrists and to the medial aspects of to the lead wire, position
electrodes on the patient’s legs
the ankles.
with the lead connections
pointing superiorly.
Expose the patient’s chest and apply a pregelled electrode at each electrode position on To guarantee the best connection
the patient’s chest. If the patient is female, be to the lead wire
sure to place the chest electrodes below the
breast tissue.
Note: disposable electrode applies directly to
the prepared site, as recommended by the
manufacturer.
56
• V1: Fourth intercostal space at right sternal
border.
• V2: Fourth intercostal space at left sternal
border.
• V3: Halfway between V2 and V4.
• V4: Fifth intercostal space at the left
midclavicular line.
• V5: Fifth intercostal space at anterior axillary
line (halfway between V4 and V6)
• V6: Fifth intercostal space at midaxillary
line, level with V4.
Ask the patient to relax and breathe
normally. Tell the patient to lie still and not
to talk when you record the ECG.
Press the AUTO or START button. Observe
the tracing quality. The machine will record
all 12 leads automatically, recording 3
consecutive leads simultaneously.
When the machine finishes recording the 12lead ECG, turn off the machine, remove the
electrodes, and clean the patient’s skin.
After disconnecting the lead wires from the
electrodes, dispose of the electrodes, as
indicated.
4)Post care
Return the bed to the lowest position
To minimize artifact
Some machines have a display
screen so you can preview
waveforms before the machine
records them on paper
To prevent falls and
maintain patient safety.
Clean and dry patient skin and cover the
patient’s arms, legs, and chest, and then
return patient to comfortable position
Remove gloves and hand washing
Clean, disinfect, and prepare the equipment
for future use.
57
Open door, window and curtains
5)Documentation
Verify the date, time, patient’s name, date of
birth assigned identification number on the
ECG itself and other relevant information,
such as symptoms that occurred during the
recording.
Document the date and time the ECG was
obtained and significant responses by the
patient in the patient’s medical record
Note any appropriate clinical information on
the ECG and place it in the patient’s medical
record.
Right electrocardiogram
An electrocardiogram (ECG) that measures the electrical activity of the
heart. Unlike a standard 12-lead ECG, used primarily to evaluate left
ventricular function, a right chest lead ECG reflects right ventricular
function and provides clues to damage or dysfunction in this chamber.
Indications for RT ECG
Right chest lead ECG for a patient with an inferior wall myocardial
infarction (MI) and suspected right ventricular involvement.
Procedure
All steps of standard ECG except chest lead site
58
V1 R (4th Intercostal space) to right of sternum.
V2 R (4th Intercostal space) to left of sternum.
V3 R directly between V1 and V4.
V4 R (5th Intercostal space) at right midclavicular line.
V5 R (5th Intercostal space) at right anterior axillary line.
V6 R (5th Intercostal space ) at right midaxillary line .
Posterior Electrocardiogram
POSTERIOR CHEST LEAD is a recording of the electrical activity of
the heart. The 12-lead ECG is an essential diagnostic tool in the
management and treatment of ischemic heart disease, but because of the
location of the heart’s posterior surface, changes associated with
myocardial damage may not be apparent on a standard 12- lead ECG. To
help identify posterior involvement, some practitioners recommend using
posterior chest leads in addition to the limb leads of the 12-lead ECG
despite lung and muscle barriers.
Indication for posterior ECG:
Posterior leads may provide clues to posterior wall infarction. Because
patients with coexisting posterior infarction are at greater risk for
59
complications so acute therapy, including thrombolysis and angioplasty,
shouldn’t be delayed.
Procedure
V1, V2, V6 at the same place at anterior chest wall.
To ensure an accurate electrocardiogram (ECG) reading, make sure
that the posterior leads V7, V8, and V9 are placed at the same
level horizontally as the V6 lead at the fifth intercostal space.
Place V6 at the midaxillary line.
Place lead V7 at the posterior axillary line, lead V9 at the left
paraspinal line, and lead V8 halfway between leads V7 and V9.
ECG Interpretation
P wave represents atrial depolarization.
The PR interval is measured from the beginning of the P wave to
the beginning of the QRS complex. The PR interval is normally
0.12 to 0.20 seconds long and represents the time it takes for the
electrical impulse to travel from the SA node to the AV node
The QRS complex represents ventricular depolarization (the time it
takes for the impulse to travel through the bundle branches to the
Purkinje fibers). QRS complex duration is normally less than 0.11
second (2.5 small boxes)
60
The ST segment is the portion of the ECG waveform that extends
from the end of the QRS complex to the beginning of the T wave.
QT interval is measured from the beginning of the QRS complex
to the end of the T wave and indicates the total time from the onset
of ventricular depolarization to the completion of ventricular
repolarization.
The U wave follows the T wave, but isn’t always seen.
Introduction:
An exercise ECG is performed to assess the heart's response to stress or
exercise. The ECG is monitored while a person is exercising on a
treadmill or stationary bike.
Definition:
Exercise stress testing is a noninvasive test. It consists of an ECG tracing
during a period of physiologic stress on the heart muscle and its blood
supply to discover and diagnose ischemia that is not apparent at rest.
61
Indications of stress ECG:
Diagnose coronary artery disease.
Causes of chest pain,shortness of breath or
lightheadedness.
Functional capacity of the heart after an MI or heart
surgery.
Effectiveness of antianginal or antiarrhythmic
medications.
Occurrence of dysrhythmias.
Contraindications of stress ECG:
Acute MI within 48 hours, unstable angina.
Uncontrolled dysrhythmias with hemodynamic compromise.
Severe aortic stenosis.
Acute myocarditis or pericarditis, and decompensated HF.
Unstable angina not yet stabilized with medication.
Severe pulmonary hypertension.
Pulmonary embolism (a clot in the arteries of a lung).
Acute renal failure –infection).
Procedure:
Physiologic stress is created by asking the patient to walk on a
treadmill or to ride a stationary bicycle.
Exercise places unique demands on the cardiovascular system.
Systemic oxygen consumption increases markedly, requiring the
heart to increase CO to meet these demands.
Myocardial contractility increases, resulting in greater SV and
systolic blood pressure. HR is increased as a result of circulating
catecholamines.
62
When the myocardial demand for oxygen increases during
exercise, coronary blood flow must increase to maintain an
adequate oxygen supply.
In patients with CAD, coronary blood flow cannot increase
sufficiently to meet the high metabolic needs of the myocardium
during exercise, and ischemia results.
Stress test protocols.
Numerous protocols have been developed using a treadmill. Two
popular ones:
Bruce protocol, in which the grade and speed are varied every 3
minutes.
Balke protocol, in which speed remains constant and grade is
gradually increased every minute.
Regardless of the protocol used, the heart rate and rhythm is
monitored continuously. Blood pressure is also measured and
recorded every minute during the test.
The diagnostic value of the test is based on the maximal HR
achieved, not on the length of time that the patient remains on the
treadmill.
Clinical reasons to stop a treadmill test.
A treadmill test may be aborted before maximal HR is reached if
symptoms occur (development of moderate to severe anginal or
chest pain).
Signs of pallor or poor perfusion, and the patient asking to stop the
test.
ST segment elevation or ST depression or cardiac dysrhythmias.
63
Increased symptoms such as breathlessness or fatigue or a fall in
blood pressure of 10 mm Hg or more from baseline.
Elevated blood pressure: blood pressure is expected to rise during
exercise, but a systolic blood pressure greater than 250 mm Hg or a
diastolic blood pressure greater than 115 mm Hg is an indication to
stop the test.
Patient preparation (nursing role)
Nursing care before stress ECG
The nurse prepares the patient for the stress test by describing how
the stress test is performed, the type of monitoring equipment
used, the rationale for insertion of an IV catheter, and what
symptoms to report.
Men should expect to have some chest hair shaved so that the
electrodes will press directly against the skin.
Prepare emergency equipment on stress ECG room (crash
car- D.C. shock-emergency medication).
The patient is instructed to fast for at least 3 hours before the test,
medications may be taken with sips of water and avoid stimulants
such as alcohol ,tobacco and caffeine.
Clothes and rubber-soled shoes suitable for exercising are to be
worn.
The primary provider may instruct the patient not to take certain
cardiac medications, such as beta-adrenergic blocking agents,
calcium channel blocker 48 hours and digoxin 2 weeks before the
test.
Avoid significant exertion on test day.
Take medication of hypertension.
Obtain base line ECG and blood pressure.
64
Nursing care during stress ECG
During an exercise stress test, the patient walks on a treadmill
(most common) or pedals a stationary bicycle. Exercise intensity
progresses according to established protocols.
Attached 10 electrodes to the person's chest and back.
The electrodes record the heart's electrical impulses and transmit
them to an electrocardiograph.
A machine that turns the impulses into waves on graph paper or on
a monitor screen.
Patient will also wear a blood pressure monitoring cuff or
monitoring device during the test. The electrodes may be placed on
top of a light layer of gel that conducts electricity and helps ensure
clear readings.
During the test, the following are monitored: 2 or more ECG leads
for heart rate, rhythm, and ischemic changes; BP; skin temperature;
physical appearance; perceived exertion; and symptoms, including
chest pain, dyspnea, dizziness, leg cramping, and fatigue. The test
is terminated when the target heart rate is achieved or if the patient
experiences signs of myocardial ischemia. Further diagnostic
testing, such as a cardiac catheterization, may be warranted if the
patient develops chest pain, extreme fatigue, a decrease in BP or
pulse rate, serious dysrhythmias, or ST-segment changes on the
ECG during the stress test.
Nursing care after stress ECG
Remove ECG electrodes and clean chest area.
Relax and reassure the patient.
Monitor ECG, blood pressure and symptoms.
65
Introduction:
Holter monitors are the most widely used continuous recording systems
for patients with daily symptoms. Holter monitoring is used to determine
how the heart responds to normal activity. This is a totally noninvasive
procedure with no adverse effects.
Definition
A Holter monitor is a battery-operated portable device that measures and
records heart’s activity (ECG) continuously for 24 to 48 hours or longer,
mainly used for patients with unpredictable cardiac symptoms that
occur in random and short episodes. The device is the size of a small
camera. It has wires with silver dollar-sized electrodes that attach to the
skin. Wearing a Holter monitor has no risks and causes no pain.
Indications of Holter monitoring:
Atrial fibrillation or flutter
Multifocal atrial tachycardia
Palpitations
Paroxysmal supraventricular tachycardia
Repeated fainting
66
Slow heart rate (bradycardia)
Ventricular tachycardia.
Procedure
The patient wears skin electrodes and carries a small box that
contains a digital recorder.
The monitor is carried by a shoulder strap or clipped to a belt or
pocket for 24 hours or longer and then is returned to the hospital or
clinic for reading.
All Holter monitors record between 3 and 12 leads to minimize
inaccurate interpretation caused by artifact. Most systems have an
event marker, which the patient can press to indicate the onset of
symptoms.
The patient is asked to keep a diary of activities, symptoms, and any
medications that are taken.
Patch monitors are a newer generation that continuously records the
ECG for up to 14 days. These are small adhesive devices that are
more convenient than Holter monitors but can only record in one or
two leads.
Activities that would get the chest electrodes or monitor wet,
including swimming and taking a shower or tub bath while wearing
a Holter monitor should be avoided.
The patient is asked to avoid electric blankets, high-voltage areas
and magnets while wearing the device
67
Introduction
Definition of dialysis
Types of dialysis
Definition of hemodialysis
Indication of hemodialysis
Contraindication of hemodialysis
Dialysis system
Hemodialysis care (pre-during-post)
Care of AV fistula or AV graft
Hemodialysis complication
68
The kidney play vital role in fluid and toxins removal and when
kidney function goes down from 10 to 15% of normal, a buildup of toxins
(waste) and fluid in body happen. Dialysis is a form of renal replacement
therapy ensures the maintenance of homeostasis (a stable internal
environment) in people experiencing a rapid loss of kidney function, i.e.,
acute kidney injury (AKI) or chronic kidney disease (CKD).
Is a form of renal replacement therapy or the kidney’s role of filtration of
the blood is supplemented by artificial equipment to remove excess water,
solutes, and toxins when patient can no longer be managed with
conservative therapies, such as diet, drugs, and fluid restriction, dialysis is
indicated.
Hemodialysis (HD)
Peritoneal dialysis (PD)
Continuous renal replacement therapy (CRRT)
69
"Hemo" means blood and "dialysis" means filter.
Is the most common renal replacement therapy used with ESKD and
kidney failure. Is a procedure which dialysis removes excess fluids and waste
products and restores chemical and electrolyte balance.
HD involves passing the patient’s blood through an artificial
semipermeable membrane to perform the filtering and excretion functions of
the kidney.
Acute kidney injury
Uremic encephalopathy
Pericarditis
Life-threatening hyperkalemia
Refractory acidosis
Hypervolemia causing end-organ complications (e.g., pulmonary edema)
Asymptomatic patients with a GFR of 5 to 9 mL/min/1.73 m²
Any toxic ingestion
70
Absolute contraindication to hemodialysis is
Inability to secure vascular access
Sever hemodynamic instability
Active bleeding
Relative contraindications include:
Difficult vascular access
Needle phobia
Cardiac failure
Coagulopathy
Hemodialysis removes wastes and water by circulating blood outside the body
through an external filter, called a dialyzer, which contain a semipermeable
membrane.
Diffusion
Diffusion’ is the term used to describe the movement of molecules from an
area of high concentration of solutes to a region of low concentration of
solutes until they are equal.
Osmosis
Movement of water across a semipermeable membrane from a solution that
has a lower osmolarity or concentration to a solution with a higher osmolarity
or concentration. Potassium and sodium typically move out of the plasma into
the dialysate. Bicarbonate and calcium generally move from the dialysate into
the plasma.
Ultrafiltration
Fluid removal is achieved by altering the hydrostatic pressure of the dialysate
compartment, causing free water and some dissolved solutes to move across
the membrane along a created pressure gradient
71
dialyzer, dialysate, vascular access routes, and
an HD machine.
1- Dialyzer The dialyzer is the functional unit of the extracorporeal circuit
just as the nephron and some patients and nurses refer to the dialyzer as the
‘kidney’ and provide highly efficient clearance of waste products. A dialyzer
has four parts: a blood compartment, a dialysate compartment, a
semipermeable membrane, and an enclosed support structure.
The core of the dialyzer is made up of thousands of tiny mesh tubes.
The blood flows inside each tube, and the dialysate stays on the outside of
the tubes.
Tiny pores in the tubes let waste and excess fluids pass from the blood
into the dialysate.
The cleaned blood then leaves the dialyzer and is returned to the body.
2-Dialysate
is made from clear water and chemicals and is free of any waste
products or drugs. Because bacteria and other organisms are too large to
pass through the membrane, dialysate is not sterile.
The water used in dialysate must meet specific standards and usually
requires special treatment before mixing the dialysate.
The dialysate composition may be altered according to the patient’s
needs for management of electrolyte imbalances. During HD, the
dialysate is warmed to 100° F (37.8° C) to increase the diffusion rate
and to prevent hypothermia.
3-The HD machine
Are artificial kidneys that perform most, but not all, kidney functions for
patients who have permanent or temporary renal failure. The machines use
hemodialysis to cleanse the blood and balance its constituents. With this
process, the patient's blood is circulated through the machine where it is
filtered and balanced for electrolytes, pH, and fluid concentration before
being returned to the patient. It has alarm systems to monitor for potential
problems
72
4- Vascular access: There are three types of accesses: Depending on patient
health, the strength of your veins, and other factors, there are different types
of access sites.
Arteriovenous (AV) fistula 4 is surgically created by connecting an
artery to a vein. It is placed in the wrist, upper arm or forearm. It is
created to cause extra pressure by pushing an increase in the blood
flow into the veins, making it stronger and bigger, thereby creating
easy vascular access to the blood vessels.
A V graft is a surgically created connection between vein and artery. It
allows direct access to the bloodstream for dialysis. Usually an arm
vein is connected to an artery
Central venous catheter it is used for emergency hemodialysis, a
plastic tube (catheter) may be inserted into a large vein in your neck
and the catheter is temporary
73
FOR HEMODIALYSIS
Access Type
Description
AV fistula
AV graft
Hemodialysis
catheter
(dual- or
triple-lumen)
Subcutan
eous
device
Location
Permanent
An internal
anastomosis of an
artery to a vein
Synthetic vessel
tubing tunneled
beneath the skin,
connecting an
artery and a vein
Initial use
Forearm
2-4 month or
longer
Forearm
Upper arm
Inner thigh
1-2 wks
Temporary
A specially designed
catheter with two
or three lumens Two
Subclavian,
lumens are for blood
internal
outflow and inflow for
jugular, or
hemodialysis; a third
femoral
allows venous access
vein
without accessing dialysis
lumens
An internal device
with two metallic
access ports and
Subclavian
two catheters
inserted into large
central veins
Immediately after
insertion and x-ray
confirmation of
placement
Immediately
after
insertion
Pre Dialysis Care
A-Care of patient before hemodialysis
-Medications such as (anticoagulant, antibiotics and vasoactive drugs )
-History of blood disorders.
-Laboratory values (CBC, PT, PTT, INR, PLTs, serum electrolytes and
virology).
74
-History of sensitivity or adverse reaction to a dialysate solution, iodine, or any
numbing medicine.
-Check vital signs especially blood pressure, Recommended pre dialysis blood
pressure should be <140/90 mmHg, and <130/80 mmHg post-dialysis.
-Check patient weight: The term ‘target weight’ or ‘ideal weight’ or dry
weight referred to the weight at which there is no clinical evidence of edema,
shortness of breath, increased jugular venous pressure or hypotension or
hypertension.
-Assess the patient’s level of consciousness to identify on any signs of
disequilibrium syndrome.
-Withdraw labs such as Na, K, Urea, and Creatinine.
-Assess AV fistula or shunt condition and access site for
1. Check shunt and palpate for thrill and auscultate for bruits for AV
fistula.
2. Assess the patient’s distal pulses and circulation in the arm with the
access.
3. Check patency of central venous catheter, if inserted. The exit site
should be examined before each dialysis and observed for signs of
infection such as soreness, redness or the presence of exudate.
B-Care of patient during hemodialysis
-Assess and document symptoms of disequilibrium syndrome (headache,
nausea, vomiting, restlessness, decreased level of consciousness, seizures and
coma) during dialysis session.
-Measure the blood pressure during the session continuously every 1 /2or 1 hour
because hypotension is a very common complication associated with
hemodialysis and is probably a reflection of the large amounts of fluid that is
removed during the procedure.
75
-In the patient being dialyzed via a fistula or graft the heparin infusion should
be switched off 30 minutes before the end of the dialysis session to prevent the
risk of bleeding from the access site post dialysis.
C-Care of patient after hemodialysis
-Closely monitor the patient immediately and for several hours after dialysis for
any side effects from the treatment.
-Obtain vital signs
-weight the patient.
-If the patient has a fever, sepsis may be present and a blood sample is needed
for culture and sensitivity.
Caring for the Patient with an Arteriovenous Fistula or
Arteriovenous Graft
Do not take blood pressure readings using the extremity in which the
vascular access is placed.
Do not perform venipuncture or start an IV line in the extremity in
which the vascular access is placed.
Palpate for thrills and auscultate for bruits every 4 hours while the
patient is awake.
The bruit and thrill should be checked regularly (half-hourly at first)
and the patients should be taught how to perform these observations as
soon as they are able.
Assess the patient’s distal pulses and circulation in the arm with the
access.
Elevate the affected extremity postoperatively.
Encourage routine range of motion exercises.
The wound site should be examined regularly for signs of excess
bleeding or swelling.
76
The blood flow through the fistula should be checked regularly
Check for bleeding at needle insertion sites.
Assess for manifestations of infection at needle sites.
Instruct the patient not to carry heavy objects or anything that compresses
the extremity in which the vascular access is placed.
Instruct the patient not to sleep with his or her body weight on top of the
extremity in which the vascular access is placed
Patients should be advised to contact the hospital immediately if they notice
bleeding, swelling or absence of bruit or thrill
Evaluate reports of pain, numbness, or tingling; note extremity swelling
distal to access as this may indicate inadequate blood supply.
Assess skin around vascular access, noting redness, swelling, local
warmth, exudate, and tenderness.
The most common complications associated with hemodialysis are:
1. Intradialytic hypotension: This causes poor long-term outcomes due to
increased mortality known as myocardial stunning. A BP lower than 90 mmHg
strongly correlates with mortality. It usually presents as dizziness, lightheadedness, and nausea.
77
Management
Maintaining the patient in the Trendelenburg position.
Rapidly administering a 100 mL bolus of normal saline through the
bloodline.
Reduce the ultrafiltration rate and observe the patient until vitals have
stabilized.
2. Muscle cramps: Hypotension, high ultrafiltration rate, hypovolemia, and lowsodium dialysis solution predispose to cramps. These factors trigger
vasoconstriction and muscle hypo-perfusion, with secondary impairment of
muscle relaxation.
Management When occurring concomitantly with hypotension
Treatment with 0.9% saline is effective.
Forced stretching of the muscle involved provide relief of cramps.
Reduce the ultrafiltration rate.
3- Dialysis disequilibrium syndrome: This is more common in patients during or
soon after their first treatment. It is a clinical syndrome characterized by
neurologic deterioration, restlessness, mental confusion, headache, occasional
muscle twitching, and coma. It occurs due to a substantial gradient between the
urea concentrations in the CSF and blood that causes water movement into the
central nervous system (CNS), resulting in raised intracranial pressure. Patients
undergoing fast dialysis develop seizures and cerebral edema more often.
Management
It is a medical emergencies and must be managed by an immediate
stopping of dialysis, clamping of lines, and supportive care followed
by definitive care
78
Adding an osmotic agent to the blood could prevent the gradient from
forming. Sodium, mannitol, high glucose dialysate, and glycerol are
usually added. Setting the dialysate’s sodium concentration higher
throughout the treatment may be beneficial.
4- Dialyzer reactions: it is anaphylactic reaction present with dyspnea, increased
body and local temperature at the fistula site, a feeling of impending doom,
itching, urticaria, watery eyes, abdominal cramping, and diarrhea. Symptoms
may begin anytime during the first 30 minutes following dialysis due to
hypersensitivity to ethylene oxide used to sterilize dialyzers.
Management
Intravenous antihistamines, steroids, and epinephrine.
Proper rinsing of dialyzers before use eliminates residual allergens and helps
prevent them.
5- Hemolysis: Acute hemolysis during dialysis is a medical emergency indicated
by the port-wine appearance in the venous blood line, a marked fall in the
hematocrit, and a pink-colored plasma centrifuged blood sample. The patient
should be evaluated by hematologic investigations and kept under observation
for delayed hemolysis. A dialysate sample must be investigated to find the
cause.
6- Air embolism: This is a fatal complication with foam noted in the dialyzer’s
venous blood line. A churning sound may be heard during chest auscultation.
Management
Place the patient in a left lateral recumbent position.
Clamp blood lines and stop blood pump.
Normal saline to support blood pressure.
Administer 100% oxygen by mask and aspirate air from the cardiac
chambers with a percutaneously inserted needle or cardiac
catheterization.
7. Vascular access dysfunction, most commonly stenosis of arteriovenous
access, is the strongest determinant of the quality of life of a dialysis
79
patient. There are reduced blood flow and risk for thrombosis. The
formation of a catheter-related fibro-epithelial sheath also hampers blood
flow.
Management
Urokinase instillation, endovascular catheter stripping, or replacement of the
indwelling dialysis catheter in a subcutaneous tunnel re-establish access.
8- Other nonspecific complications include nausea and vomiting (10%),
headache (70%), chest and back pain (1% to 4%), and itching. These are
probably related to hypotension or could be an early manifestation of
disequilibrium syndrome.
Treating the associated hypotension resolves the symptoms.
A single predialysis dose of 5 to 10 mg metoclopramide is sufficient.
Acetaminophen given during dialysis can help manage the headache.
Switching to a different type of dialyzer membrane could reduce itching
caused by low-grade hypersensitivity to blood circuit components.
80
OUTLINES
Blood Physiology
Introduction
Terminology
Indications
Contraindications
Methods of plasma separation
Complications
Nursing Care
81
Blood Physiology
Blood is a fluid that moves through the vessels of a circulatory system. The
main components of blood are:
plasma
red blood cells
white blood cells
platelets
What is plasma?
Plasma is the liquid portion of the blood that transports water and
nutrients to all the cells in the body.
It is composed of approximately 92% water, 7% proteins and 1% other
component.
Plasma contains many specialized proteins (antibodies) that aid in
fighting infections and can be used to make life saving medical
products.
82
Introduction
Plasmapheresis is a term used to refer to a broad range of procedures in
which extracorporeal separation of blood components results in a filtered
plasma product. It is a process in which plasma is separated from blood cells
and then plasma is replaced with another solution like albumin of FFP. The
underlying mechanism of this procedure is accomplished by either
centrifugation or filtration using semipermeable membranes. This intervention
results in a filtered plasma product that can be used for the treatment of
numerous diseases.
TERMINOLOGY
Apheresis:
is a medical procedure that involves removing blood from a patient,
separating the blood into its individual components, and then returning
the desired components to the patient.
Plasmapheresis
Plasmapheresis is a therapeutic intervention that involves
extracorporeal removal, return, or exchange of blood plasma.
Plasma Exchange
is another type of apheresis that involves removing the patient’s plasma
and replacing it with donor plasma or a plasma substitute.
Indications of plasamaheresis
A. Autoimmune Disorders: Plasmapheresis is often used to manage
autoimmune diseases by removing harmful antibodies or immune
complexes from the plasma.
B. Neurological Conditions: Certain neurological disorders, such as GuillainBarré syndrome or myasthenia gravis, may benefit from plasmapheresis to
reduce autoantibodies affecting the nervous system.
C. Hematological Disorders: It can be employed in conditions involving
abnormal proteins in the blood, like cryoglobulinemia or hyperviscosity
syndrome and thrombotic thrombocytopenic purpura.
D. Some types of Caners:
Lymphoblastic lymphoma: This is a blood cancer that happens when
lymphoid cells in bone marrow or lymph nodes produce unusually large
83
amounts of the antibody immunoglobulin M. Providers use plasma
exchange to filter the antibody from the plasma.
Multiple myeloma: This is a blood cancer that happens when the bone
marrow produces abnormal plasma cells that become cancerous and
multiply.
The American Society for Apheresis (ASFA)periodically revises the
indications for plasmapheresis and classifies them according to the Grading
of Recommendations Assessment, Development and Evaluation (GRADE)
criteria. The following are some of the indications, and their categorization,
from the society’s guidelines.
Category I (disorders for which apheresis is accepted as first-line therapy,
either as a primary standalone treatment or in conjunction with other
modes of treatment) are as follows:
Guillain-Barre syndrome.
Myasthenia gravis.
Chronic inflammatory demyelinating polyneuropathy.
Hyperviscosity in monoclonal gammopathies.
Thrombotic thrombocytopenic purpura.
Goodpasture syndrome (unless it is dialysis-dependent and there is no
diffuse alveolar hemorrhage).
Hemolytic uremic syndrome (atypical, due to autoantibody to factor H).
Wilson disease.
Category II (disorder for which apheresis is accepted as second-line
therapy, either as a standalone treatment alone or in conjunction with
other modes of treatment) are as follows:
Lambert-Eaton myasthenic syndrome.
Multiple sclerosis (acute central nervous system demyelination disease
unresponsive to steroids).
RBC alloimmunization in pregnancy.
Mushroom poisoning.
Acute disseminated encephalomyelitis.
84
Hemolytic uremic syndrome (atypical, due to complement factor
mutations).
Autoimmune hemolytic anemia (life-threatening cold agglutinin disease).
Systemic lupus erythematosus (severe).
Myeloma cast nephropathy.
Category III (disorders for which the optimal role of apheresis therapy is
not established; decision-making should be individualized) are as follows:
Post-transfusion purpura.
Autoimmune hemolytic anemia (warm autoimmune hemolytic anemia).
Hypertriglyceridemic pancreatitis.
Thyroid storm.
Category IV (disorders in which published evidence demonstrates or
suggests apheresis to be ineffective or harmful; institutional review board
[IRB] approval is desirable if apheresis treatment is undertaken in these
circumstances) are as follows:
Stiff person syndrome.
Hemolytic uremic syndrome (typical diarrhea-associated).
Systemic lupus erythematosus (nephritis).
Immune thrombocytopenia.
Contraindications
Non-availability of central line access or large bore peripheral lines
Hemodynamic instability or septicemia.
Severe Anemia.
Coagulation Disorders.
Allergic Reactions: to substances used in plasmapheresis )Known allergy
to fresh frozen plasma or replacement colloid/albumin. Known allergy to
heparin.
Hypocalcemia (restricts the use of citrate as an anticoagulant during the
procedure); relative contraindication.
Angiotensin-converting enzyme (ACE) inhibitor used in last 24 hours,
relative contraindication.
85
Plasma volume in a normal person is approximately 35-40 ml/kg body weight.
The lower number 35 is applicable to patients with normal HCT values, and 40 is
applicable to patients with HCT less than normal. Plasma removal during the first
session is usually 20 ml/kg and 40 ml/kg in subsequent sessions.
There is a simplified method (Kaplan s equation) for predicting the estimated
Plasma volume = }0.065 X weight(kg) X (1- Hct) [
Hematocrit = %[hematocrit]/100
Methods of Separation
1. Centrifugal plasma separation
Blood from the patient is taken into port, anticoagulant is
added (mostly citrate). Blood goes to the centrifugal cell
separation machine. Cells go peripherally and plasma
remains in the center. Plasma goes into the plasma
collector. Cells are sent back to the patient s body and
replacement fluid is given.
86
2. Membrane Plasma separation
This technique is used in the dialysis machine. Blood
drawn from the patient mixed with anticoagulant
(heparin). Blood passed through a hollow fiber plasma
filter. Plasma is filtered off and plasma is collected and
discarded. Cells along with colloid replacement forms
new blood. New blood is given back to the patient's body.
Centrifugal cell
separation
Membrane plasma filtration
The molecular
weight of the
substance
No upper limit
Membrane pore size 0.2 to 0.5
micrometer All immunoglobulins (IgG>
IgM) Size - 3 million Dalton
Blood flow
50-150 ml/min
100-300 ml/min
Access
Large bore peripheral
cannula
adverse effects
Thrombocytopenia- in 50%
of patients
Blood flow >300 ml/min- cause
hemolysis Takes longer
Anticoagulation
Citrate
Heparin
Factor
AV access (fistula or jugular cannula)
Replacement Fluid
Removal of plasma- 50 ml/kg/body weight. Plasma volume is replaced with
crystalloid or Human albumin/colloid and FFP (Fresh Frozen Plasma).
Crystalloid - normal saline, Hypoproteinemia and hypotension occur if normal
saline alone is used as replacement fluid.
Colloid – albumin. Depletion coagulopathy occurs if albumin alone is used as
replacement fluid. Can be used in all exchanges except TMA.
Fresh Frozen Plasma- To replenish coagulation factors fresh frozen plasma is
given, given at the end of the cycle. To be used when bleeding risk is high. Each
session depletes- 60% of immunoglobulins. 5 sessions deplete 90% of
87
immunoglobulins. Always plasmapheresis must
immunosuppression to control disease progression.
be
combined
with
Plasma volume= total blood volume × (1-hematocrit). Total blood volume is
75% of body weight in males and 65% of body weight in females. Plasma to be
removed= plasma volume/body weight.
Choice of replacement solution
Solution
Albumin
Fresh Frozen Plasma
Crystalloid
Solutions
Advantage
No risk of hepatitis
No concern about ABO
blood group
Allergic reactions rare
Coagulation factors
Immunoglobulins
Inexpensive
No side effects.
No risk of infection
Disadvantage
Expensive
No coagulation Factors.
No immunoglobulins
Risk of hepatitis
Allergic reactions
Must be ABO Compatible
Citrate load
Do not maintain oncotic
pressure.
Technique:
The steps for performing plasmapheresis using centrifuge-based equipment are as follows:
1. Initially, a waste of around 3-5 mL of blood from the central
venous catheter is discarded.
2. After drawing baseline samples for complete hemogram,
calcium, and fibrinogen, it is flushed with 5-10 mL of
heparinized saline.
3. The double-lumen catheter is now connected to the machine
tubing to start the priming procedure.
4. The machine calculates total body volume (TBV), and the
effective plasma volume (which equals TBV × (1 – hematocrit))
of the patient based on the operator entered height and weight.
88
5. The replacement product to be used and its desired volume (40 60 mL/kg) is decided by the clinician, and entered into the
machine, based on which it calculates the centrifuge speed.
6. The separated plasma is discarded by the machine, and the RBCs
are returned back to the patient along with the replacement fluid.
7. Finally, post-procedure, tubing are connected to heparinized
saline, and reinfusion is initiated
8. Post-plasmapheresis blood for fibrinogen and calcium is sent
again, and lumens of central venous catheters are flushed.
Complications
Related to Vascular access.
Hematoma
Pneumothorax
Retroperitoneal bleed
Related to Procedure
Hypotension
Bleeding
Edema
89
Loss of cellular elements (plts)
Hypersensitivity reactions
Related to Anticoagulation
Metabolic Alkalosis from citrate
Bleeding
Hypocalcemic Symptoms: Tingling and numbness, arrhythmias, hypotension
NURSING CARE
Before apheresis procedure
A TPE order must be checked the following information:
- Number of plasma volumes (PV) to be exchanged
- Replacement fluid to be used :(albumin volume and no of plasma
units
For TPE, the most commonly used replacement fluids are 5%
human albumin solution and plasma, including frozen plasma (FP)
or fresh frozen plasma, (FFP), also normal saline may be used in
combination with albumin or plasma.
- Vascular access site
- Anticoagulant therapy
- Frequency and estimated total number of treatments
Informed consent for apheresis requires that the patient or
substitute decision-maker is fully aware of the risks and potential
benefits of this treatment.
The Patient should be asked to avoid caffeine containing liquids
twenty four hours before procedure due to their diuretic action.
Patient should empty the bladder prior to the procedure
Comfortable clothing with loose fitting sleeves that pull easily
above the elbow will make it easier to place the needle in each arm.
90
The physical examination of the patient should include, at
minimum, vital signs, weight, and venous access (peripheral or
central)
Laboratory tests of a complete blood count; electrolytes and
creatinine;
calcium,
magnesium,
phosphate,
albumin
and
coagulation profile (PT, PC and INR) must be checked. Additional
laboratory studies may also be necessary, depending on the
indication for apheresis.
All the equipment must be available and ready to use.
During apheresis procedure
Patient
- Monitor vital signs: heart rate, blood pressure, temperature,
saturation, central venous pressure (CVP)
- Assess patient for headache, hypotension, muscle cramps,
backache, and nausea or vomiting.
-
Calcium may be infused intravenously while the patient is
undergoing
the
plasmapheresis;
in
addition,
calcium
supplementation by mouth may also be given to prevent muscle
cramps and hypocalcemia.
- Neurological status assessment
- Assess vascular access for bleeding
Apheresis machine
Complete checkup of the apheresis circuit
- Monitor pump flow rate
- Monitor for Any alarms
After apheresis procedure
-
Check vital signs
Check for any reaction
Monitor venous access site for bleeding.
Keep an accurate record of the patient replacement fluid.
Monitor patient weight.
91
Portacath care
Introduction:
A port-A-Cath is considered a central line, it is surgically implanted. An
implanted port consists of a subcutaneous injection port attached to a
catheter. The tip of the catheter is inserted in the subclavian vein and
advanced to the superior vena cava.
Definition:
An implantable port is a type of intravenous (IV) access system that is
inserted underneath the skin in the upper chest. It is sometimes called a
Portacath or TIVAD (totally implanted venous access device). It consists
of a fine tube, or catheter, connected to a small chamber with a selfsealing silicone membrane providing easy access to one of larger veins.
Advantage:
Prevention and reduction of infection in the line and ensures the
portacath can be used for many years if required.
92
Patients with very small veins which can be damaged with the
chemotherapy drugs, or have difficult access, making a port ideal
for them.
Easy and quick access with less pain than typical needle sticks.
Longevity of device use.
Low-maintenance care at home
Body image (not noticeable under the skin)
Able to give multiple treatments at the same time (i.e. blood and
antibiotics)
Nothing external when the port is not in use
Purpose
Collect blood samples.
Intravenous (IV) medication such as anesthesia and some types of
chemotherapy, must go through a large vein.
IV fluids.
IV blood products, such as platelets and plasma.
IV contrast.
Nutritional feeding.
Long term IV medications.
Contraindication
Skin infection, skin rash, or a newly tattooed area.
Extensive scarring from burns, surgery, injuries, repeated
venipuncture, or trauma.
Hematoma
Lymphedema: The upper extremity on the ipsilateral side of a
mastectomy should be avoided owing to the frequent presence of
lymphedema occurring after dissection and removal of the
lymphatic system
93
Types of Ports
A port can be single or double-lumen.
Single-lumen ports are most common and
typically sufficient for patients requiring
scheduled intravenous therapy.
A double-lumen port is advantageous for
patients who often receive multiple
intravenous therapies at once. If two
intravenous agents aren’t compatible in the
same line, you can infuse both
simultaneously in different port lumens without complication.
-The double-lumen port also allows a concurrent infusion of
medication, chemotherapy, blood products, or parenteral nutrition.
It is also beneficial for drawing labs without interruption of an
infusion.
A Power Port is a special type of port, available in single or
double-lumen, which can withstand higher injection pressures. This
is an important consideration for receiving intravenous CT contrast
dye. A Power Port must be accessed with a particular type of
needle, a PowerLoc needle, in order to inject contrast.
A single-lumen port is a port with 1 access point.
A double-lumen port is a port with 2 access points. A needle can be
put in each access point.
94
The portal chamber is always characterized by a triangular-shaped
body, which can be palpated under the skin. In addition, a patient with
a PowerPort will receive a wallet-sized identification, keyring card,
and bracelet. It is helpful for patients to carry one or all of these
identifiers to help healthcare professionals in the future appropriately
access and utilize the PowerPort.
Location of the port
The surgeon determines the location of the port on the body based on a
patient’s internal anatomy or personal preference. It is most often
placed under the subcutaneous tissue of the chest, upper arm, or lower rib
cage.
95
Procedure
Steps
Rational
A. Assessment
1. Patient's medical record:
Physician order
Patient’s name, age and diagnosis.
Patient’s medications especially
anticoagulant.
History of:
- Blood
disorders
and
lab
investigations such as PT, PTT and
INR.
- Allergy to the antiseptic, anesthetic,
or prescribed solution.
- Length of time the port has been in
- If the port has been placed recently,
assess surgical incision. Note presence of
Steri-Strips, approximation, ecchymosis,
redness, edema, and/or drainage.
place.
Prescribed medication (ten rights).
Special consideration according to
different types of medications.
Blood test as ordered
2. patient assessment
The level of consciousness,
- To determine the need for sedation or
paralytic agents.
level of anxiety
Assess patient skin for any
96
swelling, redness, or drainage
Assess patient level of pain
B-Preparation
- To save time and effort.
1. Prepare equipment
• Sterile tape or Steri-Strips
•
Sterile
semipermeable
transparent
dressing
• Several 2 x 2 gauzes
• Sterile towel or drape
• 2% chlorhexidine solution
• Normal saline (NSS) vial and 10-mL
syringe or prefilled 10-mL NSS syringe.
• Heparin 100 U/mL in 10-mL syringe or
10 U/mL/kg.
• Noncoring needle (Huber needle) of
appropriate length and gauge
• Masks (2)
• Clean gloves
• Sterile gloves
• Additional PPE, as indicated
• Skin protectant wipe
• Alcohol wipe
• Povidone-iodine wipes
• Positive pressure end cap
• IV securement/stabilization device, as
appropriate
• Bath blanket
97
2- For self:
Hand washing.
-To prevent cross infection
Put on disposable gloves.
Wear PPE if needed
3- For environment:
Close doors, windows and pull
curtains.
- To maintain patient privacy.
Ensure adequate lighting.
Maintain bed at high comfortable
position at waist level to ensure
body mechanics.
Place a waste receptacle or bag at
a convenient location for use
during the procedure.
4. For patient:
Check the patient identification.
Explain The procedure & reason for
portacath care
Put patient in comfortable position
that provides easy access to the port
site.
C) Implementation
Needle insertion
1. Use the bath blanket to cover any - Use of a bath blanket provide for comfort
exposed area other than the site.
and warmth.
2. Put on clean gloves.
98
- Palpate the location of the port and - Knowledge of location and boundaries of
assess site.
port are necessary to safely access site.
- Note the status of any surgical
incisions that may be present.
- Remove gloves and discard.
3- Prepare a sterile field, apply sterile
gloves and wear PPE. Apply sterile
drape.
4- Connect the end cap to the extension
tubing on the non-coring needle.
5.
Clean end cap with alcohol wipe.
- Priming extension tubing removes air
Insert syringe with normal saline into from tubing and prevents administration of
end cap.
air when connected to port.
- Fill extension tubing with normal
saline and apply clamp.
- Place on sterile field.
6. Using the chlorhexidine swab
- Site care and replacement of
or povidone-iodine, cleanse the
dressing are accomplished using
port site. Press the applicator
sterile technique. Organisms on the
against the skin.
skin can be introduced into the
- Apply chlorhexidine using a
tissues or the bloodstream with the
back and forth friction scrub for at
needle.
least 30 seconds.
- Chlorhexidine is effective against
- Moving outward from the site,
the most common causes of catheter99
use a circular, scrubbing motion to
associated central line infections
continue to clean, covering at least
a 2- to 3-inch area.
-
Do not wipe or blot.
- Allow to dry completely.
7. Using the nondominant hand,
-
Hold
the
port
with
your
locate the port. Hold the port
nondominant hand so that the needle
stable, keeping the skin taut (The
is inserted into the port with the
edges of the port must be palpated
dominant hand
so that the needle can be inserted
into the center of the port).
8. Visualize the center of the port.
- To function properly, the needle must be
Pick up the needle. Coil extension
located in the middle of the port and
tubing into palm of hand. Holding
inserted to the back of the port.
needle at a 90-degree angle to the
skin, insert through the skin into
the port septum.
9. Pull back on the syringe
Positive blood return confirms patency
plunger to aspirate for blood
before administration of medications and
return.
solutions
Aspirate
only
a
few
milliliters of blood; do not allow
blood to enter the syringe
100
10. Insert the syringe with normal
saline.
Open
the
clamp
- If needle is not inserted correctly, fluid
on
will leak into tissue, causing the tissue
extension tubing and flush with 3
to
to 5 mL of saline, while observing
infiltration. Flushing without resistance
the
is also a sign that the needle is inserted
site
for
fluid
leak
or
infiltration. It should flush easily,
swell
and
producing
signs
of
correctly.
without resistance. Don’t forcibly
flush the device
11- Remove syringe. Insert heparin
syringe and instill the solution over 1
minute or according to facility policy.
12. Remove syringe and clamp the
extension tubing.
- Alternately, if IV fluid infusion is to be
started, do not flush with heparin.
13. Apply the skin protectant to the site, - Skin protectant improves adhesion of
avoiding direct application to needle dressing and protects skin from damage and
insertion site. Allow to dry.
irritation when dressing is removed.
14. Apply tape or Steri-Strips in a star- - Secures needle to help prevent the needle
like pattern over the needle to secure it. from accidentally pulling out.
Support the wings of the noncoring
needle (if present) with sterile gauze.
101
15. Apply transparent site dressing or - Dressing prevents contamination of the IV
securement/stabilization
device, catheter and protects insertion site.
centering over insertion site.
16. Label dressing with date, time of - Labeling helps ensure communication
change, and initials of IV fluid infusion
about venous access site dressing
is ordered.
change.
102
Needle removal
1. Put on gloves. Stabilize port needle
with nondominant hand.
2. Clean the end cap on the extension
tubing
and
insert
the
saline-filled
syringe.
-
Unclamp the extension tubing and
flush with a minimum of 10 mL of
normal saline
3. Remove the syringe and insert the
heparin-filled syringe, flushing with 5
mL heparin (100 U/mL or per facility
policy).
- Remove syringe and clamp the
extension tubing
4. Gently pull back transparent dressing,
beginning with edges and proceeding
around the edge of the dressing.
Carefully remove all the tape that is
securing the needle in place
5. Secure the port on either side with the
fingers of your nondominant hand.
6. Grasp the needle/wings with the
fingers of dominant hand. Firmly and
smoothly, pull the needle straight up at a
90-degree angle from the skin to remove
it from the septum .Engage needle guard,
103
if not automatic on removal.
7. Apply gentle pressure with the gauze
to the insertion site.
- Apply a Band-Aid over the port if any
oozing occurs. Otherwise, a dressing is
not necessary.
D) Post care:
Return
patient
to
comfortable To prevent cross infection.
position.
Open doors, windows and re- open
curtains.
Return equipment.
Remove gloves and hand washing.
E) Documentation:
The location of the port and the size
of needle used to access the port.
signs and symptoms of infection or
trauma
patient’s reaction to the procedure
if the patient is experiencing any pain
or discomfort related to the port
Medication (name and dose)
104
Unexpected outcome
Port begins to swell when flushing with saline: Stop flushing.
Verify that needle is against the back of the septum. Attempt to
flush. If swelling persists, stop flush. Remove needle. Obtain
additional supplies and reaccess port with new needle. Check
blood return and flush. If swelling persists, stop flush. Depending
on facility policy, leave access needle in place. Cover with
transparent dressing. Notify primary care provider. Anticipate
diagnostic tests to determine patency of port.
Port does not flush: Check clamp to make sure it is open. Gently
push down on needle and again try to flush. Ask the patient to
perform a Valsalva maneuver. Try having the patient change
position or place the affected arm over the head, or try raising or
lowering the head of the bed. If the port still does not flush,
remove needle. Obtain additional supplies and reaccess with new
needle. Check blood return and flush. If still unable to flush,
notify primary care provider. Depending on facility policy, leave
access needle in place. Cover with transparent dressing.
Anticipate diagnostic tests to determine patency of port.
Port flushes but does not have a blood return: Ask the patient to
perform a Valsalva maneuver. Try having the patient change
position or place the affected arm over the head, or try raising or
lowering the head of the bed. If the port still does not have a
blood return, remove needle. Obtain additional supplies and
reaccess with new needle. Check blood return and flush. If it still
does not have a blood return, notify primary care provider.
Depending on facility policy, leave access needle in place. Cover
with transparent dressing. Anticipate diagnostic tests to determine
patency of the port and/or instillation of thrombolytic.
105
SPECIAL CONSIDERATIONS
When port is accessed and not being used for infusion, flush daily
with 5 mL of heparin (patients weighing more than 10 kg, use 100
U/mL of heparin; patients weighing less than 10 kg, use 10 U/mL of
heparin) according to facility policy .
Groshong devices do not require the use of heparin for flushing.
Heparin-induced thrombocytopenia (HIT) has been reported with the
use of heparin flush solutions. Monitor all patients closely for signs
and symptoms of HIT. If present or suspected, discontinue heparin.
Monitor platelet counts for patient receiving heparin flush solution
when there is an increased risk of HIT.
No dressing is needed when the port is not accessed.
If the access site becomes red, becomes painful, or has yellow-green
drainage, contact your health-care provider immediately.
Patency is maintained by periodic flushing. The length and gauge of
the needle used to access the port should be selected based on the
patient’s anatomy, amount of subcutaneous tissue at the site, and
anticipated infusion requirements. In general, a 3 ⁄4-inch 20-gauge
needle is frequently used. If the patient has a significant amount
longer length (1 or 1.5 inch) may be selected. A larger gauge (19gauge) is preferred for administration of blood products.
Complication
Infection.
Damage to the port
Dislodgement of the port
Irritation with certain clothes
Occlusion or blockage
Hematoma formation.
Catheter thrombosis
106
Extravasation: the unintentional leakage of vesicant fluids or medications
from the vein into the surrounding tissue
Vesicant: agents capable of causing blistering, tissue sloughing or
necrosis
These drugs can be sub-classified according to mechanism of action by
which they cause damage, but which is also important in that it affects
the management strategy.
• DNA – binding: these drugs are absorbed locally, enter cells and bind
to their DNA, precipitating the death of the cell. Following this, the drug
can then be re-released to further destroy healthy cells leading to deeper
erosion of cells within the tissue
• Non-DNA-binding: These drugs initiate cell death by mechanisms
other than binding DNA and are eventually metabolized in the tissue and
are more easily neutralized than the DNA-binding vesicants. Injuries
resulting from these agents generally remain localized and improve over
time
Non-Vesicants: Inert or neutral compounds that do not cause
inflammation or damage. Do not cause ulceration, however they do tend
to cause pain at, and around the injection site, and along the vein.
107
Causes of extravasation
Diagnosis:
Patients must be informed to report any changes in sensation, signs,
or
symptoms
during
the
IV
administration
of
any
chemotherapeutic drug and to alert the healthcare professionals to
early signs of extravasation. Extravasation must be suspected if
any of the following specific signs or symptoms are presented
In the case of peripheral IV catheter
- Possibly no initial symptoms of extravasation
- Redness, pruritus, and edema around the injection site
- Fluid injection rate slows down or stops
- Blood backflow does not work well or there is leakage of
medication around the needle
- A complaint of discomfort or pain and occasional expression of
searing pain or numbness
- Initial physical symptoms usually appear immediately but also
might appear several days or weeks later.
In the case of central venous catheter
- Often causes stinging pain
108
- Edema around the port insertion or in the chest, or medication
leakage around the catheter insertion
- Redness in the chest, collarbone, or neck where a central venous
catheter is inserted
- No blood backflow
- Symptoms may appear early or late.
Assessment
Initial Acute assessment
A site assessment should be conducted every hour when there are
fluids or medications running through the line. If nothing is being
infused, the site should be assessed before accessing the line and
at least every eight hours.
109
Prevention of extravasion
Vesicant agents should be admininsterd by qualified personal(Only
staff who have completed appropriate training should administer
chemotherapy unsupervised.)
Cannulating over joints, the anticubital fossa or dorsum of the hand
should be avoided when administering chemotherapy.
Use a large vein in the forearm for peripheral vesicant administration.
A new cannula ideally should be placed prior to the administration of
chemotherapy. There is an increased risk of extravasation if a
previously placed cannula is utilised.
It is recommended that the smallest gauge cannula is placed in the
biggest vein possible
The vascular access device should be secured utilising a clear
dressing that enables visibility whilst maintaining adequate fixation.
Infusion lines must be secured effectively, however never cover the
line with a bandage as the insertion point must be visible at all times.
For infusion of vesicant drugs of longer duration (e.g. 12-24h) central
venous access is highly recommended.
Patients should be informed of the importance of reporting
immediately any change in sensation, stinging or burning during the
administration of chemotherapy
Regularly check the extravasation kit and refill any used medications.
Extravasation kit includes the following: 25G needle, 10-cc
syringe, and 1-mL syringe; disinfection swabs, sterile gauze, and
adhesive bandage; saline solution (1 ampule); sterile distilled water (1
ampule); dimethyl sulfoxide 99% solution; hyaluronidase 1,500
U/mL (refrigerated); hydrocortisone cream 1%; sodium thiosulfate
25% solution; and warm pack and an ice pack (frozen).
110
Note :
Treatment of extravasation
111
At the first sign of extravasation, the following steps are
recommended:
(1) Stop administration of IV fluids immediately.
(2) Disconnect the IV tube from the cannula.
(3) Aspirate any residual drug from the cannula.
(4) Administer a drug-specific antidote.
(5) Notify the physician
Elevation of the limb may aid in reabsorption of the infiltrate or
extravasated vesicant by decreasing capillary hydrostatic pressure. Apply
sterile dressing over the area of extravasation, regularly assess the
extravasation site during every shift, and take medical photographs and
consult the department of cosmetic surgery if necessary.
Thermal application
Local thermal treatments are used to decrease the site reaction and
absorption of the infiltrate. Local cooling (ice packs) aids in
vasoconstriction, theoretically limiting the drug dispersion. Cold
application is recommended for extravasation of DNA-binding vesicants
except for mechlorethamine (nitrogen mustard), contrast media, and
hyperosmolar fluids.
The use of local warming therapy (dry heat) is based on the theory that it
enhances vasodilation, thus enhancing the dispersion of the vesicant agent
and decreasing drug accumulation in the local tissue.
The use of local warming is recommended for the extravasation of non–
DNA-binding
vesicants.
Although
clear
benefit
has
not
been
demonstrated with thermal applications, it remains a standard supportive
112
care, and the recommended application schedule for both warm and cold
applications is 15 to 20 minutes, every 4 hours, for 24 to 48 hours.
Local cooling
- It causes contraction of blood vessels, minimizing the spread of drugs to
other tissues and reducing topical infections and pain.
- Directions: apply cold fomentations for 15 to 20 minutes four to 6 times
per day (for 1 day or more).
Local warming
- It dilates the blood vessels around the extravasation site, increases
dispersion and absorption of the medicinal fluid by increasing the blood
flow, and helps to quickly purge medicinal fluid that has leaked from the
extravasation site.
- Directions: apply hot fomentations for 20 to 30 minutes four to 6 times
per day (for 1 day or more).
Documentation:
Because errors associated with IV administration can result in fatal or
life-threatening outcomes, administration of IV fluids and medications
can be a high-risk, with adverse outcomes potentially leading to
malpractice claims.
An incident of extravasation must be correctly documented and reported.
Documentation procedure may differ between treatment centers
(documentation form); however, certain items are mandatory for patient
safety and legal purposes: (1) patient name and number, (2) date and time
113
of the extravasation, (3) name of the drug extravasated and the diluent
used (if applicable), (4) signs and symptoms (also reported by the
patient), (5) description of the IV access, (6) extravasation area (and the
approximate amount of the drug extravasated), and (7) management steps
with time and date.
Photographic documentation can be helpful for follow-up procedures.
The patient must be informed of the scope of the problem
114
115
Miao, J., Krisanapan, P., Tangpanithandee, S., Thongprayoon, C., &
Cheungpasitporn,
W.
(2024).
Efficacy
of
Therapeutic
Apheresis
for
Cryoglobulinemic Vasculitis Patients with Renal Involvement: A Systematic
Review. Blood Purification, 53(1), 1-9.
Kaplan, A. A. (2008). Therapeutic plasma exchange: core curriculum 2008. American
Journal of Kidney Diseases, 52(6), 1180-1196.
Fernández-Zarzoso, M., Gómez-Seguí, I., & de la Rubia, J. (2019). Therapeutic
plasma exchange: review of current indications. Transfusion and Apheresis
Science, 58(3), 247-253.
MEDICAL-SURGICAL NURSING: ISBN (single volume): (2-volume set): 978-14377-2799-9 Copyright © 2013, 2010, 2006, 2002, 1999, 1995, 1991 by
Saunders, an imprint of Elsevier Inc. Copyright Licensing Agency, can be
found at our website: www.elsevier.com/permissions.
Ernstmeyer K and Christman E, (2023). Nursing Advanced Skills. 2nd edition ,
Chippewa Valley Technical College,USA, chapter 5,pp
Bloom L & Seckel MA, (2022). Placement of nasogastric feeding tube and
postinsertion care review. AACN Advanced Critical Care, 33(1), 68–84.
Perry, A.G., & Potter ,P.A., & Ostendorf ,W.R.(2019): Mosby's boket
guide To Nursing Skills & Procedures .9th ed.pp.(93-109).
Nettina, S.M., (2019): Lippincott Manual Of Nursing Practice.11 th ed.
PP.(531-535) (577-583).
Lynn, p.(2019):Taylor’s Clinical Nursing Skills:A Nursing Process
Approach.Bowel Elimination.5th ed..pp.(2529-2557).
116
Achi, o., (2020): Manual of Nursing Procedures and Practice.2nd ed. p.p.
(423-427)
Hagberg, c., (2023): Hagberg and Benumof's Airway Management.
Prioxygenation Fifth Edition.ch.15. p.p (276-298)
Lynn, P. (2019). Taylor’s Clinical Nursing Skills: a nursing process
approach, 5th ed., Chapter 14 (Oxygenation), China: Wolters Kluwer,
PP. 2511-2529.
117