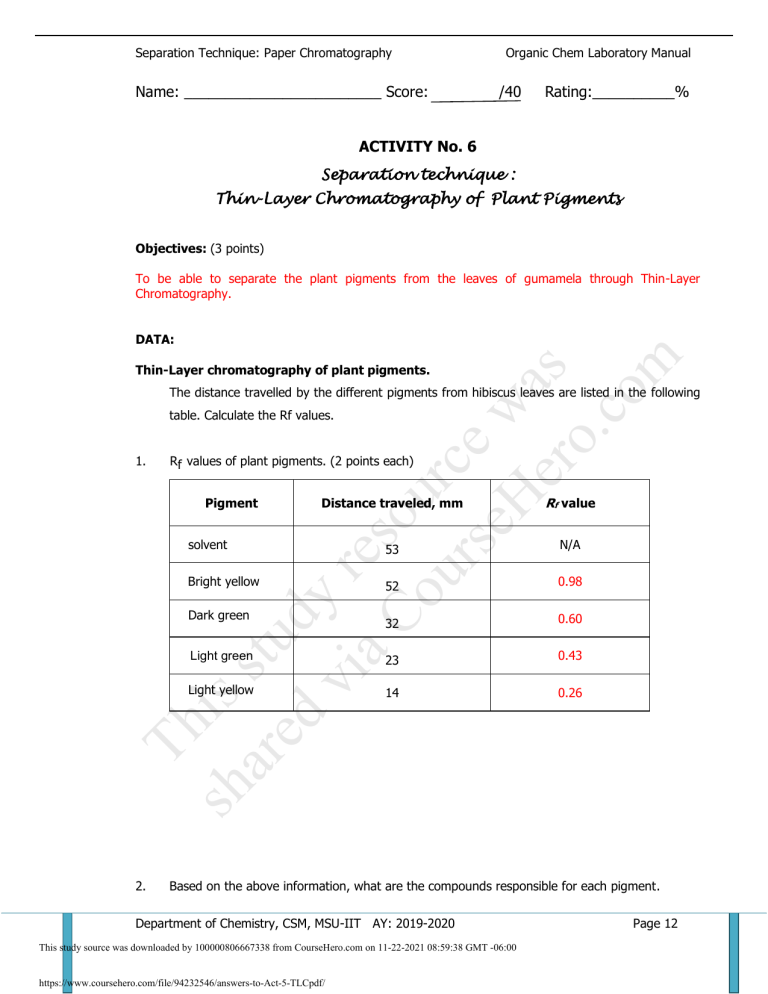
Separation Technique: Paper Chromatography Name: ________________________ Score: Organic Chem Laboratory Manual /40 Rating:__________% ACTIVITY No. 6 Separation technique : Thin-Layer Chromatography of Plant Pigments Objectives: (3 points) To be able to separate the plant pigments from the leaves of gumamela through Thin-Layer Chromatography. is ar stu ed d vi y re aC s o ou urc rs e eH w er as o. co m DATA: Thin-Layer chromatography of plant pigments. The distance travelled by the different pigments from hibiscus leaves are listed in the following table. Calculate the Rf values. 1. Rf values of plant pigments. (2 points each) Distance traveled, mm Rf value solvent 53 N/A Bright yellow 52 0.98 32 0.60 Light green 23 0.43 Light yellow 14 0.26 Pigment sh Th Dark green 2. Based on the above information, what are the compounds responsible for each pigment. Department of Chemistry, CSM, MSU-IIT AY: 2019-2020 This study source was downloaded by 100000806667338 from CourseHero.com on 11-22-2021 08:59:38 GMT -06:00 https://www.coursehero.com/file/94232546/answers-to-Act-5-TLCpdf/ Page 12 Separation Technique: Paper Chromatography Organic Chem Laboratory Manual (2 points each) Color Compound Bright yellow carotene Dark green Chlorophyll a Light green Chlorophyll b Light yellow Lutein or xanthophylls QUESTIONS: a) Which pigment is the most soluble in the developing solvent? (1 pt.) is ar stu ed d vi y re aC s o ou urc rs e eH w er as o. co m 1. Bright yellow b) Explain. (2 points) since it has the greatest affinity to the nonpolar solvent system. 2. What does a small Rf value indicate with respect to its polarity? (2 pts ) Small Rf indicates that the compound is polar since it is held more strongly to the polar stationary phase. 3. If the developing solvent is changed, would the Rf be the same? (yes or No) (1pt) Explain (2 pts) because Rf value depends on the interaction of the eluent with mobile (solvent) and stationary phase. Non-polar compounds move up the plate most rapidly (higher Rf value) with the nonpolar solvent. Is it possible to have an Rf value greater than 1? Why or why not? (3 pts) Th 4. No, because the distance travelled by the spots could not be greater than the distance 5. sh travelled by the solvent since it is the solvent that carries the spots along. The experimental results show that leaves contain different pigments, explain why we only see green color. (3 pts) The food-making process takes place in the leaf in numerous cells containing chlorophyll, which gives the leaf its green color. Most of the year the other colors are masked by great amounts of green coloring. Department of Chemistry, CSM, MSU-IIT AY: 2019-2020 This study source was downloaded by 100000806667338 from CourseHero.com on 11-22-2021 08:59:38 GMT -06:00 https://www.coursehero.com/file/94232546/answers-to-Act-5-TLCpdf/ Page 13 Separation Technique: Paper Chromatography 6. Organic Chem Laboratory Manual What is the developing solvent used? Indicate the ratio. (1 pt) 75% pet. ether: 25% acetone. 7. Is it polar or nonpolar? Underline (1 pt) 8. What is the stationary phase? (1 pt) silica 9. Is it polar or nonpolar? Underline (1pt) 10. From the chromatographic results, arrange the different pigments according to increasing polarity. (3 pts) sh Th is ar stu ed d vi y re aC s o ou urc rs e eH w er as o. co m Bright yellow < dark green < light green < light yellow Department of Chemistry, CSM, MSU-IIT AY: 2019-2020 This study source was downloaded by 100000806667338 from CourseHero.com on 11-22-2021 08:59:38 GMT -06:00 https://www.coursehero.com/file/94232546/answers-to-Act-5-TLCpdf/ Powered by TCPDF (www.tcpdf.org) Page 14