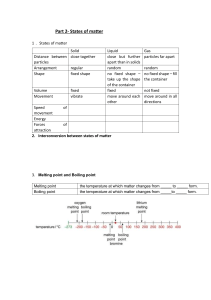
GRAND INTERNATIONAL SCHOOL IGCSE Combined Science (0653) Chemistry C1: STATES OF MATTER Learning objectives: 1. State the distinguishing properties of solids, liquids and gases 2. Describe the structures of solids, liquids and gases in terms of particle separation, arrangement and motion. 3. Describe changes of state in terms of melting, boiling, evaporating, freezing and condensing, 4. Describe the effects of temperature and pressure on the volume of a gas. 5. Explain changes of state in terms of kinetic particle theory. Introduction o all substances that exist in our universe are made up of matter. o matter: any substance that has mass and occupies space. o Chemistry is the study of how matter behaves and how one substance can be changed into another. o all substances exist in three different forms (physical states) called states of matter. These are: 1. solids (s). 3. gases (g). 2. liquids (l). s, l, g are called state symbols o the three physical states show the differences in the way they respond to changes in temperature and pressure. o The kinetic theory of matter: helps to explain the way in which matter behaves. o the theory states that: 1. all matter is made up of tiny, moving particles, invisible to the naked eye. that are in random molecular motion. Different substances have different types of particles (atoms, molecules or ions) which have different sizes. 2. The particles move all the time. The higher the temperature, the faster they move on average. 3. Heavier particles move more slowly than lighter ones at a given temperature. o The kinetic theory can be used as a scientific model to explain how the arrangement of particles relates to the physical properties of matter in terms of the movement of its constituent particles and different energies involved. 1.1: Structure and properties of solids, liquids and gases A: Solids o fixed volume and definite shape at given temperature. o particles tightly arranged together in a fixed position due to stronger intermolecular forces. o high density. o does not flow. o they are incompressible. o negligible/no space between particles. B: Liquids o has a fixed volume, but no definite shape and size. o take shape of container into which it is poured. o particles close to each, but can slide past each other easily due to weaker intermolecular forces which makes liquids flow easily. o moderate to high density. o more compressible in nature than solids and less compressible than gases. o more space between particles than solids. C: Gases o no fixed volume, shape or size. o spread out (expands) to fill container/space they are contained in, due to very weak intermolecular forces. o low density. o more compressible in nature than solids and liquids. o more space between particles than solids and liquids. Summary: Flow chart of the nature of matter Differences in properties of states of matter. Changes in physical state o changes in temperature and pressure leads to physical changes in matter that are more than contraction or expansion. o at atmospheric pressure, changes happen by either lowering or raising temperature. 1. Melting and freezing o melting point (m.p): temperature at which a substance (solid) turns into a liquid at a particular temperature. o freezing point (f.p): reverse process of melting point - temperature at which a liquid changes to a solid. - freezing happens at the same temperature with melting (melting and freezing of pure water takes place at °C, example: Gallium is a metal with melting point slightly above room temperature and causes it to melt in a person’s hand 2. Sublimation o the direct change of state from solid to gas or gas to solid through bypassing the liquid phase. o examples: few solids, such as solid carbon dioxide, do not melt when they are heated at normal pressures. Instead, they turn directly into gas. Solid carbon dioxide is often called ‘dry ice’ because the surface of the block is dry. o iodine sublimes and produces a purple vapour but condenses again on a cold surface. o this is different to a normal ice cube, which has a thin film of liquid water on the surface. 3. Evaporation, boiling and condensation o evaporation: a process occurring at the surface of a liquid, involving the change of state from a liquid into a vapour at a temperature below the boiling point. o liquid with surface exposed to the air evaporates. o larger surface area, speeds up evaporation. o warmer liquids, evaporates faster. o hot climate in Dead Sea means water evaporates easily and the sea has high salt concentration boiling: change of state in which at a certain temperature, the liquid becomes hot enough to escape from liquid (body/surface) and change to gas molecules. boiling point: the temperature at which a liquid boils, when the pressure of the gas created above the liquid equals atmospheric pressure. volatility: the property of how easily a liquid evaporates water evaporates easily, has low boiling point (100 °C). water is a volatile liquid. Ethanol, with a boiling point of 78 °C, is more volatile than water. It has a higher volatility than water and evaporates more easily condensation: the change of a vapour or a gas into a liquid and during this process, heat is given out to the surroundings (cooling happens). - it is the reverse process of evaporation. - gas state most affected by changes in pressure. It is possible, at normal temperatures, to condense a gas into a liquid by increasing the pressure, without cooling. Consider a kettle shown below. o colourless, invisible water vapour escapes from the kettle. o water vapour is present in the clear region we can see at the mouth of the kettle. o visible cloud of steam is made up of droplets of liquid o water formed by condensation as vapour cools in air. o a beaker of boiling water, form bubbles when there are enough high-energy water molecules to give a gas with a pressure equal to atmospheric pressure. o boiling point of a liquid change if surrounding pressure changes. o value given for boiling point is stated at the pressure of the atmosphere at sea level (atmospheric pressure or standard pressure). o if surrounding pressure falls, boiling point falls. o boiling point of water at standard pressure is 100 °C. o on high mountain, boiling point is lower than 100 °C. o if surrounding pressure is increased, the boiling point rises. o Photocopy pages 7 & 8: Chemistry for Cambridge IGCSE COURSEBOOK Heating and cooling curves o melting point of solids can be measured using the apparatus shown. o powdered solid put in narrow melting-point tube for easy heating. o water bath used to measure melting points ˂ 100 °C. o oil bath used to measure melting points ˃ 100 °C. o on heating, temperature rises and stays constant until all solid has melted. o temperature then rises as liquid warms further. o continuous heating of liquid in same apparatus can be done to reach boiling point. o temperature stays same until all liquid has evaporated. o experiment can be performed in reverse using similar apparatus to produce a cooling curve, but thermometer placed in test-tube with solid studied. o solid is then melted completely and liquid heated. o heating is then stopped, temperature is noted every minute as substance cools. This produces a cooling curve (Figure 1.10). The level (horizontal) part of the curve occurs where liquid freezes, forming solid. o experiments show that heat energy is needed to change a solid into a liquid, or a liquid into a gas. o During the reverse processes, heat energy given out. o Photocopy pages 9,10 & 11: EXPERIMENTAL SKILLS 1.1 Chemistry for Cambridge IGCSE COURSEBOOK 1.2 Kinetic particle theory of matter Existence of atoms and molecules o elements, compounds react to produce substances around world (e.g. massive objects – gas giants such as Jupiter, Saturn). o matter made up of very small particles called atoms. o key ideas: 1. each element composed of its own type of atoms. 2. atoms of different elements combine to makes molecules of compounds Main points of the kinetic particle theory o matter made of small particles (different substances have different particles, atoms, molecules, ions). o particles in random motion (increase in temperature, the higher the average energy of particles). o movement and arrangement of particles is different for the three states of matter. o pressure of gas produced by atoms/molecules of gas hitting the walls increase as more particles collide with the walls. Summary of particle arrangement o Refer to properties of solids, liquids, gases (1.1). o fluid properties are produced due to ability of particles to move in the liquid and gas phases. o particles: widely spread in gas, close together in liquid or gas. o intermolecular space: space between atoms or molecules in a gas or liquid. o intermolecular spaces larger in gases and reduced by increasing external pressure (gases compressible). o intermolecular spaces smaller in liquids (liquids not very compressible). Pressure, temperature and volume changes 1. pressure and volume (Boyle's Law) o increase in external pressure decrease volume, gas contracts reduces in volume. o decrease in external pressure increase volume, gas expands, increases volume. Boyle’s Law: the volume of a given mass of a gas is inversely related to its pressure when its temperature is kept constant. 2. Volume and temperature (Charles' Law) o increase in temperature of gas increases the volume of gas. Gas expands. o decrease in temperature of gas decreases volume, of gas. Gas contracts. Charle’s Law: The volume of a given fixed mass of a dry gas is directly proportional to its absolute temperature at a constant pressure. Summary on Gas Laws o volume of gas easily changed by conditions of temperature and pressure because space between rapidly moving particles is greater than liquids and solids. o temperature increase: gas particles moves faster, freely, less interaction, greater volume. o temperature lowered: particles move slowly, more likely interact with each other, move together to occupy small volume. o increase in pressure: particles close together causing them to interact more. o decrease in pressure: particles occupy greater space, interaction between particles is less. Differences between evaporation and boiling Evaporation Boiling o happens at all positions in the liquid at once because all particles have enough kinetic energy to move faster and turn to a gas. happens at surface of liquid because not all but only some particles have enough kinetic energy to move faster and leave surface of liquid and turn into a gas. o happens at lower temperatures/any happens at higher/specific/fixed temperature temperatures called boiling points o temperature may changes temperature remains constant o slow process quick process o no bubbles formed bubbles formed Interpretation of a cooling curve o arrangement of particles in states of matter, help to explain energy changes involved when a substance is heated/ cooled. cooling of gas (region A): o temperature falling, gas cools. o energy of particles decreasing. o particles move slowly, interact more strongly, become close to form liquid. o Intermolecular forces between particles increase, heat given out (exothermic). o results in temperature becoming constant until gas condenses to liquid. cooling of liquid (region B): o temperature falling, liquid cools. o energy of particles decreasing. o particles more slow, strong interaction, liquid becomes solid. o Intermolecular forces between particles increase, heat is given (exothermic). o solid forming, release of energy keeps temperature constant until freezing ends formation of solid (region C): o after solid forms, temperature falls again. o particles in solid vibrate less strongly as temperature falls. Key points (freezing and condensation) 1. when particles come closer together, new forces of interaction occur. 2. energy is given out. 3. temperature constant until liquid or solid is totally formed. NB: freezing/condensation are exothermic reactions Heating curve o when an experiment is carried out in the opposite direction, from solid to liquid forms a heating curve. o temperature constant during melting/ boiling. o energy absorbed to break bonds /overcome forces between particles for free movement (endothermic) Key words: o intermolecular forces: the attractive forces that act between molecules. o exothermic changes: process/chemical reaction in which heat energy is produced and releases to surroundings. Enthalpy for this change is negative: o endothermic changes: process/chemical reaction that takes heat from the surroundings. Enthalpy for this change is positive. 1.3 Mixtures of substances and diffusion o chemical world is complex: made up of a mixture of different substances joined together. o mixture – two or more substances joined together, not chemically bonded but separated by physical means. - no chemical reaction (no new substance). - physical change (substance formed change from one state to another). - the three states can combine in many ways: e.g states completely mix to make one state/phase o solution – mixture formed when a solute (solid substance) is dissolved in a solvent (solvent). o example: solid salt completely dissolve in liquid water to produce a liquid mixture called salt solution. o solute – a solid substance that dissolves in the liquid (solvent) to form a solution. o solvent – a liquid substance that dissolves the solid (solute) to form a solution. o solution = solute + solvent o water is a common solvent, liquids that act as solvents in organic chemistry: organic solvents. o suspension – mixture in which one phase is broken down to small particles of insoluble solid, droplets or bubbles spread throughout the main phase (liquid). o example: fine particles of a solid in a liquid after a precipitation reaction. o precipitation reaction – reaction in which an insoluble salt is made from solutions of two soluble salts. Solutions o Earth’s surface made of a mixture of solids and solvents. o examples: - 2/3 of surface covered with salts dissolved in sea water. - dissolved gases oxygen and carbon dioxide important for sea life exists in oceans. o Common solvents are: 1. water. 2. organic solvents: ethanol, propanone, trichloroethane. o Why are organic solvents important they dissolve substances that do not dissolve in water. o soluble – a solute that dissolves in a particular solvent. o insoluble – a substance that does not dissolves in a particular solvent. o miscible – two liquids form a completely uniform mixture when combined. o alloys – mixture made by joining liquid metals and solidifying them. - designed to have properties for a certain use. solder = tin + lid (low melting point). Examples of miscible/immiscible substances Miscible Immiscible o water + milk water + oil o water + ethanol water + kerosene o water + vinegar water + benzene o water + lemon juice honey + oil Solubility of solids in liquids o dissolving Copper (II) Sulfate in fixed volume of water, solution becomes more concentrated as more solid Copper (II) Sulfate is added. o concentrated solution: higher proportion of solute, small proportion of solvent (solute > solvent). o dilute solution (less concentrated): small proportion of solute, high proportion of solvent (solvent > solute). o saturated solution: more solid solute is added and a point is reached when no more solute dissolves in solvent at constant temperature. - solid dissolves when temperature is increased. o concentration: measure of how much solute is dissolved in solvent to make a solution. o concentration of solute in saturated solution is the solubility of the solute at that temperature. o solubility of solids depends on temperature e.g crystallization. Solubility of gases in liquids o gases less soluble in water as temperature rises. o solubility from air in water small, but dissolved oxygen is enough to support aquatic life. o solubility of gases increase with pressure. o example: sparkling drinks contain carbon dioxide dissolved under pressure. o fizzing happens due to pressure release when opening lid, container left open drink goes flat.