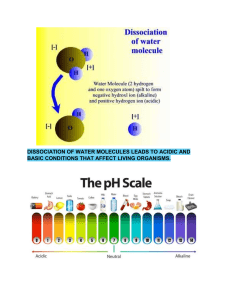
Acid-Base equilibria Ionic equilibrium refers to the state of balance that is achieved in a solution of an electrolyte, in which the concentration of the ions in the solution remains constant over time. In other words, ionic equilibrium is the condition in which the rate of ionization of an electrolyte is equal to the rate of recombination of the ions that are produced. OR It is the state of a reversible reaction in which the rate of forward reaction equals the rate of the backward reaction and the concentration of reactants and products remain the same. In an ionic equilibrium, the electrolyte may exist in both ionized and unionized forms, depending on the concentration of the ions in the solution. The equilibrium is described by an equilibrium constant, which is a measure of the ratio of the concentration of the products to the concentration of the reactants at equilibrium. Ionic equilibrium is important in a variety of fields, including chemistry, biochemistry, and environmental science. It is used to understand the behavior of acids and bases in solution, the solubility of salts, the transport of ions in biological systems, and the chemical reactions that occur in the atmosphere and the ocean. Degree of dissociation / ionisation The degree of dissociation, symbolized as α (alpha), is a term used in chemistry to describe the extent to which a compound dissociates or breaks apart into its constituent ions or molecules in a solution. OR Number of moles dissociated per mole of reactant. The degree of dissociation is defined as the ratio of the number of dissociated particles (ions or molecules) to the initial number of particles in the solution. This ratio is usually expressed as a fraction or percentage. Factors that can influence the degree of dissociation of a compound include: 1. Nature of the compound: The chemical nature of the compound can affect its ability to dissociate in a solution. Compounds with strong ionic or covalent bonds are less likely to dissociate than those with weaker bonds. 2. Concentration of the solution: The degree of dissociation tends to increase with increasing concentration of the solution. This is because there are more opportunities for the ions or molecules to interact with each other and with the solvent molecules. 3. Temperature: Increasing the temperature of the solution can increase the degree of dissociation. This is because higher temperatures can break down the bonds between the ions or molecules, allowing them to dissociate more easily. 4. Pressure: Changes in pressure can also affect the degree of dissociation for gases that dissolve in liquids. An increase in pressure tends to increase the solubility of a gas in a liquid, which can increase the degree of dissociation. 5. Presence of other ions: The presence of other ions in the solution can affect the degree of dissociation of a compound. For example, if a solution contains a large amount of ions that can form a complex with one of the dissociated ions of the compound, then the degree of dissociation can be reduced. Ionic Equilibria and Degree of Dissociation (Scope: dissociation of electrolytes in aqueous solution, degree of dissociation: definition, factors, derivation and statement of Ostwald’s dilution law, calculations). Ionic equilibria and degree of dissociation are important topics in chemistry, particularly in the study of electrolytes in aqueous solutions. The degree of dissociation, symbolized as α (alpha), is a term used to describe the extent to which a compound dissociates or breaks apart into its constituent ions in a solution. When an electrolyte is dissolved in water, it dissociates into its constituent ions. The degree of dissociation is defined as the ratio of the number of dissociated particles (ions or molecules) to the initial number of particles in the solution. This ratio is usually expressed as a fraction or percentage. Factors that can influence the degree of dissociation of a compound include the nature of the compound, concentration of the solution, temperature, pressure, and the presence of other ions. Ostwald’s dilution law is a statement that relates the degree of dissociation of a weak electrolyte to the concentration of the solution. According to this law, the degree of dissociation of a weak electrolyte is directly proportional to the square root of the concentration of the solution. The law can be derived from the expression for the equilibrium constant for the dissociation of a weak electrolyte. Calculations related to the degree of dissociation can be used to determine the pH of a solution or to predict the solubility of a salt. For example, the degree of dissociation of a weak acid can be used to calculate the pH of a solution using the acid dissociation constant (Ka). Similarly, the degree of dissociation of a salt can be used to predict its solubility in water. Overall, the study of ionic equilibria and the degree of dissociation is important in understanding the behavior of electrolytes in aqueous solutions, and has applications in a wide range of fields, including medicine, agriculture, and industry.