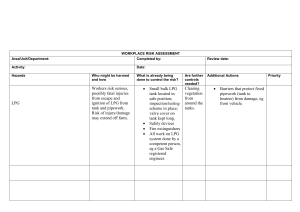
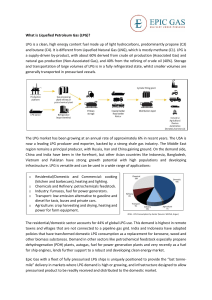
THE EVALUATION AND DESIGN OF LPG TREATERS Jenny Tse and Jesse Santos The Dow Chemical Company GAS/SPEC Technology Group Freeport, Texas Presented at 43rd Annual Laurance Reid Gas Conditioning Conference March 1-3, 1993 Norman, Oklahoma INTRODUCTION Liquid-liquid extraction is a process used in the purification of liquefied petroleum gas (LPG) or natural gas liquid (NGL) streams. Purification involves the removal of acid gas contaminates such as hydrogen sulfide (H2S), carbon dioxide (CO2), and other sulfur species from liquid hydrocarbon streams containing C2 to C4 compounds. One common method of acid gas removal is by extraction with an aqueous caustic solution that is discarded when H2S or CO2 is removed. However, this method is expensive because of the associated chemical and disposal costs. An alternative method is extraction with an alkanolamine that forms a weak regenerable salt. These alkanolamines include monoethanolamine (MEA), diethanolamine (DEA), methyldiethanolamine (MDEA), and formulated MDEA solvents. This paper reviews the criteria for designing LPG treaters which use alkanolamines as extraction solvents. Plant case studies of typical LPG treater designs will provide insight into the physical and chemical aspects dominating the overall mass transfer in these systems. Finally, these criteria will be employed to troubleshoot an LPG treater. OVERVIEW OF LIQUID EXTRACTION WITH ALKANOLAMINES "Liquid-liquid extraction consists of the following steps: (1) intimate contacting of solvent with the component to be extracted so that the solute will be transferred from the solution to the solvent and (2) separation of two immisible phases."[1] In the case of LPG treating, the acid gases (the solutes) are removed from the LPG by contacting the solution with an amine (the solvent). LPG treating can be considered a special case of extraction where the mass transfer is accompanied by chemical reactions. This phenomenon is composed of the following steps [1]: (1) Diffusion of the solute from the bulk of the organic phase to the organicaqueous interface. (2) Diffusion of solute from the organic-aqueous interface to the bulk aqueous phase. (3) Chemical reaction within the aqueous phase. (4) Diffusion of the reaction products due to the concentration gradient. FLOW CONFIGURATION In LPG treating, the aqueous amine phase is usually the continuous phase, while the organic LPG phase is usually the dispersed phase. The aqueous amine solution is fed to the top of the column, while the LPG feed enters from the bottom. Once contact is complete and the phases separate, the rich amine containing the acid gases will exit the bottom of the column as the extract. The raffinate, or the treated LPG, will exit from the top of the column. Figure 1 illustrates the flow configuration. Figure 1. Flow configuration for LPG treating with an amine. The selection of the LPG as the dispersed phase and the amine as the continuous phases provide several advantages in terms of mass transfer: (1) When the phase with the highest flowrate is dispersed, the total surface area available for mass transfer is maximized. [2,3] (2) Dispersion of the LPG into the higher viscosity amine phase reduces the terminal velocity of the droplets which increase the droplet residence time and hence the mass transfer. EQUIPMENT SELECTION Three types of devices are commonly used for liquid extraction with alkanolamines. The devices include packed columns, sieve tray columns and static mixers. Of these, the packed and sieve tray columns are the most prevalent, but static mixers have been used successfully in a number of applications. The selection of equipment depends on the treating requirement and the specific needs of the operation. Static mixers have been used successfully in a number of application when less than one theoretical stage is required and unit size is crucial. Packed columns and sieve tray columns are used when 2 to 7 theoretical stages are needed.[3] Each devices offer its own unique advantages and disadvantages. Laddha and Degaleesan [1] in addition to Treybel [2] have presented reviews on each of these devices. This paper will focus specifically on the design of packed and sieve tray columns. AMINE SELECTION The selection of the amine depends on the type of acid gas components that need to be removed and the specification required. A primary amine such as MEA has a high affinity for acid gases. This property makes MEA the better choice in applications where both H2S and CO2 must be removed to very low levels or where the acid gas partial pressures are extremely low. One drawback, however, is that MEA reacts irreversibly with carbonyl sulfide (COS) to form nonregenerable degradation products that reduce the acid-gas removal capacity of the amine. In Gases with high concentrations of COS, a secondary amine such as DEA should be considered because it can remove the COS without forming the nonregeneable products.[4] If the outlet specification cannot be met with a secondary amine, a final caustic scrub of the LPG may be required to remove the last traces of COS. MDEA should be used when H2S selectivity and little COS removal are required. Finally, formulated MDEA solvents can be used in varying applications. The choice of solvent may also be influenced by other factors including energy requirements and solution corrosivity. Several references offer reviews on these topics. [5,6] In general, MEA is more corrosive and requires more energy for regeneration than DEA and MDEA. PACKED COLUMN DESIGN Column Diameter and Capacity Column diameter or capacity is determined by the maximum hydraulic flow through the column or the flood point of the tower. Several investigators, including Crawford and Wilke [7] have reported correlations to estimate the flooding velocities in a packed column. In Table 1, the actual and design velocities of three operating LPG treaters were compared to the predicted flooding velocities. A modified Crawford-Wilke correlation [8] was used in this evaluation to predict the column capacity. The recommended design criterion for this correlation is 20% of predicted flood.[10] Commercial experience with systems removing H2S in LPG treaters confirmed the predicted flooding point. [3] The results from Table 1 indicate that the LPG treaters chosen are operating at a fraction of the predicted flood, ranging from 5 to 22%. Even though Plant 2 and Plant 3 have exceeded their original design flow rates, the calculations show that these treaters may still be operating well below flood. The calculation also indicates that Plant 1 is approaching maximum capacity with the dispersed phase at 22% of predicted flood velocity. Additional data need to be gathered from Plant 1 to confirm its capacity. While the above correlations should be used for design calculations, a rough estimate of the column diameter or the column capacity can be determined by the combined amine and hydrocarbon flow rate. Literature references recommend that for design the combined flow should not exceed 15 8pm per square foot of column cross sectional area.[9] A survey of several plants in Table 1 indicates that the actual combined flow ranged from 16.2 to as high as 35.6 gpm/ft 2. Both Plants 2 and 3 have original design flow of less than 15 gpm/ft 2. Plant 1, however, has a design flow of 55.7 gpm/ft 2. Plant 1 is of particular interest because the treater has never operated at its designed capacity. Excessive amine carryover was reported at an amine circulation rate of 200 8pm with an equivalent flow to diameter ratio of 37.6 gpm/ft 2. Table 1. Operating and design conditions of three packed LPG treaters. Type and Size of Packing The function of packing is to increase the mass transfer rate in the column. The packing accomplishes this function in three ways. First, it serves to increase the residence time of the droplets in the column. The total mass transfer is increased if the droplets must travel the tortuous paths of the packing interstices. Second, the packing reduces the likelihood of back-mixing within the column. Third, the packing will distort, coalesce and re-disperse the droplets to enhance internal circulation and refreshes the film surface for mass transfer.[3] The types of packing generally selected for LPG treating are either metal or ceramic. Ceramic packings are preferentially wetted by the aqueous amine solution, while metal packing will be wetted by either the aqueous or organic phase depending on initial exposure of the metal surface.[3] Since the dispersed phase is the LPG and the continuous phase is the amine, the packing should be wetted by the continuous phase. The packing should not be wetted by the LPG phase because the droplets will coalesce on the surface of the packing, reducing the efficiency. Plastic packing will also sacrifice the LPG droplet integrity and is therefore never specified for LPG treaters. The packing size is selected on the basis of efficiency and capacity. If the interstitial void is large, the efficiency will decrease due to channeling at the walls of the tower. Conversely, if the packing is too small, the capacity of the column is diminished. For optimum performance, the packing size should be greater than the critical packing size (dFC). Calculation for the critical packing size can be found in Perry's Chemical Engineers' Handbook [10]. The critical packing size can be calculated from the following relation developed by Gaylor and Pratt: Packing larger than the critical size will allow the droplets to move freely within the packing interstices, independent of the flowrate.[1,3,10] Otherwise, if the voids are too small, the packing will facilitate the coalescence of the droplets into larger drops and reduce the column capacity. For the cases listed in Table 1, the critical packing size is less than 1/2 inch. Therefore, the packing should be greater than 1/2 inch. However, for commercial applications, packing usually range from 1-1/2 to 2 inches. Packing size should be no greater than 1/8 the tower diameter to prevent channeling. [3,10,11] TRAYED COLUMN DESIGN The following section gives some design criteria for sieve tray LPG treaters. Examples of three operating LPG treaters are given in Table 2. For Plants 1 and 2, not enough data was available to compare the operating conditions with these criteria. However, adequate data was available for Plant 3. The troubleshooting section of this paper will give a detailed comparison of the operating conditions of Plant 3 with these theoretical design criteria. Table 2. Operating design conditions of three sieve tray LPG treaters. Capacity and Flood Point The capacity of sieve tray columns is also limited on consideration of the flood point. Flooding occurs when the flow rate of the continuous phase is so high that the dispersed phase accumulates under the plates. If the height of the coalescing layer exceeds the length of the downcomer, entrainment of the dispersed phase into the downcomer will occur. When the thickness of the coalescing layer approaches the plate spacing, the treating capacity of the LPG treater will be diminished. Tray Design Unlike a packed column in which dispersion and coalescence occur somewhere within the packing, dispersion and coalescence occur at every tray in a sieve tray column. Therefore, for best efficiency, the sieve trays should be designed such that the droplets will break cleanly from the hole to form the proper sized droplets. Mayfield and Church recommend that the liquid be allowed to jet and then form droplets a slight distance away from the holes.[12] The recommended average velocity throughout the holes should be between 0.5 to 1.0 ft/s. [10] To ensure that the droplets do not coalesce after they are formed, the pitch, or the distance between the holes must be kept 3 to 4 hole diameters apart.[1] The holes are usually 1/8 to 1/4 inch in diameter, set 1/2 to 3/4 inch apart, on square or triangular pitch.[1,10] Tray Spacing The tray spacing is based on the height of the coalescing layer. The accumulation of the dispersed liquid is calculated by the pressure drop required for the movement of the liquid through the holes. The total minimum liquid head required may be expressed by the following equation [1]: ht = hN + hg+ hc where, ht = total minimum head required for movement of dispersed phase, ft h N = head required to overcome the friction through the holes, ft hg = head required to overcome interfacial effects, ft hc = head required to overcome the effects of the flow of the continuous phase, ft. Detailed calculation of each parameter can be found in Reference [1] and [5]. Once the dispersed phase thickness is determined, the tray spacing can be calculated. For commercial units, 10 to 12 times the coalescing layer height is adopted to provide flexibility and to allow for the location of the manhole. In actual practice, the tray spacing range from 6 inches to 2 feet. [1] LPG/AMINE DISTIRIBUTORS AND RE-DISTRIBUTORS In both packed and sieve tray columns, proper distributions of the phases affect the tower hydraulics and performance, as well as the amine and LPG losses. Well-designed distributors will evenly disperse the liquids to provide good contact, while minimizing both losses due to entrainment and the formation of emulsions. While many types of designs are available, the following discussion will be limited to the simplest orifice type distributors used in packed columns. Proper distribution of the LPG is especially critical for packed columns to avoid mal-distribution and poor efficiency. For packing larger than the critical packing size, the droplets have a characteristic drop size when the liquids are in concentration equilibrium.[1,10] The characteristic drop size is given by the following equation.[10] If the droplets formed by the distributor are too large, the packing will breakdown these droplets until they reach their critical size.[1] Extremely large droplets will reduce the efficiency of the column by decreasing the surface area available for mass transfer. On the other hand, if the droplets formed at the distributor orifices are smaller than the characteristic drop size, there may be a tendency to flood until the drops grow to size.[10] Stable emulsion may also form if the droplets cannot quickly coalesce. The droplets' sizes are controlled by the velocity in which the LPG is dispersed and the size of the distributor orifice. The velocity at the orifice should be limited to 70 ft/min (1.25 ft/s). Orifice sizes of 0.19 to 0.25 inches in diameter are commonly used, although sizes as small as 0.14 to 0.31 inch diameter have also been successful. If the dispersed phase superficial velocity exceeds 130 ft/min, the number of orifices should be increased rather than the orifice size.[3] Flooding of the tower caused by poor dispersed phase hydraulics at the bottom of the packed bed can be avoided in two ways. The dispersed phase distributor should be embedded in the packing itself to avoid liquid hold-up at the bottom of the packed column.[3] However, if the column requires more than one bed, a redistributor and a second bed disperser plate should be designed to assure dispersed phase hold-up will not occur between the beds. A different way of approaching this problem is to couple the primary distributor with a disperser/packing support plate. While the distributor's function is to introduce the dispersed phase across the disperser/packing support plate, the disperser plate should be designed to develop the final droplet size. If this is done, risers should be placed on the distributor to extend to about 1 inch above the downcomer exit on the disperser plate for the continuous phase. Redispersion after 6 to 10 feet of packing is recommended to improve column efficiency.[3] The amine distributor should be designed to minimize turbulence which may disrupt phase separation at the top of the column. The continuous phase velocity should be no greater than 170 ft/min (2.8 ft/s). If the superficial velocities-of the continuous phase exceed 60 ft/h (1 ft/min), additional downcomers should be installed at the disperser plate. [3] TROUBLESHOOTING CASE STUDY A sieve tray column was employed in treating a C3/C4 liquid hydrocarbon stream with MDEA in a refinery (See Table 2, Case 3). The new LPG treater had no apparent problems during startup. However, after severaI weeks of operation, plant personnel noticed severe emulsion formation in the contactor. The emulsion resulted in poor phase separation of the LPG from the amine. The consequent entrainment of liquid hydrocarbons into the regenerator had resulted in unstable operation. Examination of the operating condition such as temperature, pressure, flow rate, etc., revealed nothing unusual that would cause such severe emulsification of the phases. However, a closer look at the equipment revealed several inconsistencies in the designs. The actual operating range was calculated and compared to the criteria for a typical sieve tray column (See Table 3). Table 3. Comparison of actual operating conditions versus a typical design. The most notable items in this comparison were the high velocities at the orifices of the distributors. Excessive hydrocarbon velocity was believed to be the primary cause of the emulsion. At velocities of this magnitude, the LPG exited the distributor as unsteady jet streams rather than controlled flows. The breakdown of the turbulent stream resulted in the formation of non-uniform droplets. In addition, the high velocity stream sheared through the aqueous phase to produce even smaller droplets that created a very broad drop size distribution. From a geometry standpoint, smaller aqueous droplets can exist in the interstices of the larger LPG drops to develop more stable emulsions. Further examination of the design revealed the direction in which the LPG exited the distributor to also have an impact on LPG losses. Since the distributor exit holes were pointed down, the LPG was expected to go some distance downward before the density difference overrode and the droplets reversed direction. The distance between the distributor and the tower bottom is approximately 10.5 ft. At high velocities, some hydrocarbon was apparently carried out with the rich amine. The high entrance velocity of the amine posed a concern from an amine loss standpoint. Although 8 to 10 minutes of hydrocarbon retention time above the amine distributor was allowed in the design, the feed amine velocities disturbed the amine-hydrocarbon interface and hindered separation. As a result of these findings, the refiner had additional holes drilled into both distributors to reduce the LPG and the amine entrance velocities. Since the trays effectively re-disperse the droplets, the size of the orifice diameter was not critical. To prevent possible hydrocarbon entrainment into the rich amine, the LPG distributor was inverted so that the holes were pointed in the upward direction. The equipment modification appeared to have corrected the problem and eliminated the formation of emulsion. The second startup of the plant went smoothly with no carry-over of the LPG into the regenerator. CONCLUSION The purpose of this paper is to offer some basic criteria for the design and evaluation of LPG treaters. The paper also discussed some of the rationale behind the criteria to give a better understanding of their effects on mass transfer and on the operation of LPG treaters. Actual operating data from several plants showed that these criteria can be used with reasonable reliability for evaluation purposes. In almost all the cases, the LPG treaters were built with high degrees of conservatism in their design. REFERENCES [1] Laddha, G.S. and Degaleesan, T.E., Transport Phenomena in Liquid Extraction, McGraw-Hill, 1978. [2] Treybal, R.E., Liquid Extraction, 2nd Edition, McGraw-Hill, 1963, 1978. [3] Stringle, R.F., Random Packings and Packed Towers -Design and Applications, Gulf Publishing, 1987. [4] Gas Conditioning Fact Boot The Dow Chemical Company, Midland, Michigan, 1962. [5] DuPart, M.S., Bacon, T.R., and Edwards, D.J., "Understanding and Preventing Corrosion in Alkanolamine Gas Treating Plants," Proceedings of the 1991 Gas Conditioning Conference. [6] Cross, C. Edwards, D., Santos, J., and Stewart, E., "Gas Treating Through Accurate Process Modeling of Specialty Amine Plants," Proceedings of the 1990 Gas Conditioning Conference. [7] Crawford, J.W. and Wilke, C.R., Chem. Eng. Prog., Vol. 47, 1951. [8] Nemanaitis, R.R., Eckert, J.S., Foote, E.H., and Rollinson, L.R., Chem. Eng. Prog., Vol. 67, 1971. [9] Perry, C.R., "Treating System for Ethane Recovery Plants," Proceedings of the 1977 Gas Conditioning Conference. [1 O] Perry's Chemical Engineers' Handbook, 6th edition, McGraw-Hill, 1984. [11] DuPart, M.S. and Marchant, B.D.; "Natural Liquid Treating Options and Experiences," Proceedings of the 1989 Gas Conditioning Conference. [12] Mayfield, F.D. and Church, W.L., "Liquid-Liquid Extractor Design," Ind. En=. Chem., Vol. 44, 1952.