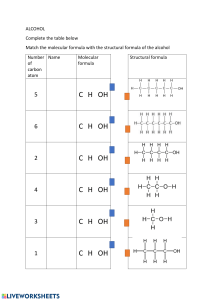
Lecture 18: Understanding Molecular Structures and Drawing Techniques Q1: Review of Molecular Geometry ● Definition: Molecular geometry refers to the three-dimensional arrangement of atoms within a molecule. ● Important Takeaways: ● Determines the molecule's physical and chemical properties. ● Predicted using VSEPR theory (Valence Shell Electron Pair Repulsion theory). ● Key Information: ● Linear, bent, trigonal planar, tetrahedral, trigonal bipyramidal, and octahedral are common geometries. ● The geometry influences molecular polarity and reactivity. How to Use Molview to Draw 3D Molecular Structure (Optional) ● Key Information: Molview is an online tool to visualize and draw 3D molecular structures. ● Important Takeaways: Useful for understanding spatial arrangements and molecular geometry. Q2: Drawing Skeletal Structures and Video Solutions ● Definition: Skeletal structure is a simplified organic molecule drawing that omits hydrogen atoms bonded to carbons. ● Important Takeaways: ● Carbons are represented as junctions or ends of lines. ● Hydrogens bonded to carbons are assumed but not shown. ● Key Examples: ● Acetic Acid, Capsaicin, 2'-deoxycytidine, etc. ● Each example highlights the process of drawing complex organic molecules. Q3: Converting Skeletal Structure to Molecular Formula ● Definition: This process involves counting the number of each type of atom in a skeletal structure to determine its molecular formula. ● Important Takeaways: Essential for understanding the composition of a molecule. Q4: Memorize Alkane with 1 to 10 Carbon Chains ● Key Information: ● Alkanes are hydrocarbons with single bonds only. ● Naming is based on the number of carbon atoms (Methane, Ethane, Propane, etc.). ● Formulas: General formula for alkanes is CnH2n+2 Lecture 19: Organic Chemistry Fundamentals Alkane, Alkene, Alkyne ● Definitions: ● Alkane: Hydrocarbons with single bonds. ● Alkene: Hydrocarbons with at least one double bond. ● Alkyne: Hydrocarbons with at least one triple bond. ● Important Takeaways: The type of bond significantly affects the chemical reactivity and properties of these molecules. Aromatics ● Definition: Aromatic compounds contain ring structures with delocalized π electrons. ● Key Information: Benzene is the simplest aromatic compound. Alcohol and Ether ● Definition: ● Alcohol: Organic compound with a hydroxyl (-OH) group. ● Ether: Compounds with an oxygen atom connected to two alkyl or aryl groups. ● Important Takeaways: Both are important solvent and functional group in organic synthesis. Aldehyde and Ketone ● Definition: ● Aldehyde: Contains a carbonyl group (C=O)bonded to at least one hydrogen atom. ● Ketone: Carbonyl group bonded to two carbon atoms. ● Key Information: Reactivity and applications in synthesis. Carboxylic Acid and Esters ● Definition: ● Carboxylic Acid: Organic acids with a carboxyl group (COOH). ● Esters: Derived from carboxylic acids and alcohols. ● Important Takeaways: Essential in biochemical processes and industrial applications. Amine and Amide ● Definition: ● Amine: Organic compounds and functional groups containing a basic nitrogen atom with a lone pair. ● Amide: Compounds with a carbonyl group linked to a nitrogen atom. ● Key Information: Amines and amides play crucial roles in biochemistry and polymer industry. Haloalkanes ● Definition: Alkanes in which one or more hydrogen atoms have been replaced by a halogen (F, Cl, Br, I). ● Important Takeaways: Useful as solvents, refrigerants, and in organic synthesis. Functional Groups in Biological System ● Key Information: Understanding functional groups is crucial to biochemistry, as they dictate molecular interaction and functionality. ● Important Takeaways: Recognition of functional groups aids in predicting molecular behavior in biological systems. Lecture 18: Mastery of Molecular Structures and Visualization Techniques This lecture delves into the foundational aspects of molecular geometry, emphasizing the three-dimensional arrangement of atoms in a molecule and how this influences chemical behavior and physical properties. It introduces tools like Molview for visualizing 3D molecular structures, offering a practical approach to understanding spatial arrangements. The lecture progresses to teach drawing techniques, specifically the skeletal structure representation, which is a streamlined method for depicting organic molecules. This section is enriched with video solutions for drawing complex molecules such as Acetic Acid, Capsaicin, and 2'-deoxycytidine, demonstrating step-by-step processes. The conversion of skeletal structures to molecular formulas is discussed, underscoring its importance in comprehending molecular composition. Lastly, memorization techniques for alkanes with 1 to 10 carbon chains are shared, highlighting the significance of hydrocarbons in organic chemistry. Lecture 19: Introduction to Organic Chemistry and Functional Groups Lecture 19 introduces the basic building blocks of organic chemistry: alkanes, alkenes, and alkynes, detailing their structures, naming conventions, and the implications of their single, double, and triple bonds, respectively. The concept of aromatic compounds is explored, with benzene presented as the quintessential example, leading to a discussion on the stability and reactions of aromatic systems. The lecture further covers essential functional groups, including alcohols, ethers, aldehydes, ketones, carboxylic acids, esters, amines, amides, and haloalkanes. Each functional group is defined, with explanations on their structural characteristics, reactivity, and role in synthetic and biological systems. A special focus is placed on the functional groups' relevance to biological systems, emphasizing how these molecular features dictate interactions and functionality within living organisms. Both lectures are structured to provide a comprehensive overview of critical concepts in chemistry, from the basics of molecular geometry to the complexities of organic functional groups. Through a combination of theoretical understanding and practical applications, these lectures aim to equip students with the knowledge and skills necessary for further exploration and study in the field of chemistry.