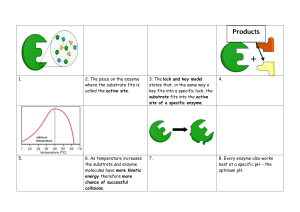
Enclonar, Kimberly / MLS 3A Enzymes Lyases Catalyze removal of groups from substrates without hydrolysis; products contain double bonds Isomerase Catalyze interconversion of geometric, optical, or positional isomers Ligases Catalyzes the joining of 2 substrate molecules February 10, 2021 Glenn Charls Buelis, RMT, MLS (ASPIi) Enzymes • Specific biologic proteins that catalyze biochemical reaction • Not consumed in a reaction • The substances in the reaction are converted into products • Act on substrate • Have high specificity for their substrate o Enzyme-Substrate relationship • Present in trace amounts in blood and are subject to saturation; released from degraded cells o Enzymes are located intracellularly • Found on all body tissues; found in serum in high concentration after cellular injury Terms to Remember • Active Site o Often a water-free cavity, where the substrate interacts with a particular charged amino acid residues • Allosteric Site o A cavity other than the active site • Isoenzyme o Different form of a particular enzyme; based on certain properties (electrophoretic ability, solubility) o Based on location • Isoform o Result when an enzyme is subject to posttranslational modifications o Removal or addition of a component • Zymogen/Proenzyme o Structurally inactive form of enzyme • Cofactor o Non-protein molecule; necessary for enzyme activity o Activator - Inorganic cofactors, such as Cl or Mg o Coenzyme - organic cofactor, such as NAD • Prosthetic Group o Coenzyme when bound tightly to the enzyme o Apoenzyme - enzyme portion o Holoenzyme - complete and active system (APOENZYME+COENZYME) Enzyme Nomenclature • An enzyme's name usually corresponds with its substrate and ends with the suffix -ase • All enzymes are given numerical designation by the EC of the International Union of Biochemistry o EC 3.1.3.1 ALP o EC 1.1.1.27 LDH • 1st number - Classification • 2nd 2 numbers - subclass & sub-subclass • Last number - serial number Enzyme Classification (OTHLIL) Oxidoreductase Catalyze oxidation and reduction reaction between 2 substrates Transferases Catalyze transfer of a group other than hydrogen from one substrate to another Hydrolases Catalyzes hydrolysis of various bonds Catalytic Reaction of Enzymes • Lowers the activation energy that the reactants must reach for the reaction to occur o Activation energy/Free energy of activation energy required to start a reaction • Rate of Reaction depends on activation energy/free energy of activation Enzyme Kinetics • Lock and Key Model o High degree of similarity between the active site and substrate • Induced-fit model o Binding of the substrate induces a conformational change in the enzyme Factors that Influence Enzymatic Reaction Substrate Concentration • General enzyme, substrate, product relationship o E + S → ES → E + P • Substrate readily binds to a free enzyme at a ↓ substrate concentration • Michaelis-Menten formula - a curve that shows the relationship of reaction velocity to substrate concentration • Adding a substrate, ↑ rate of reaction, except if all enzymes are saturated Enclonar, Kimberly / MLS 3A • First Order Kinetics o The reaction rate varies directly with the substrate's concentration with a fixed amount of enzyme • Zero order Kinetics o The velocity of reaction is not affected by the addition of more substrate at a certain point. When maximum velocity is reached, the rate increase in velocity is zero (zero order reaction). Thus, rate of reaction depends on enzyme concentration. • Endpoint (Fixed-time method) o Combines reactants, stops the reaction at a fixed time and then measures the product formed • Kinetic (Continuous monitoring) o Combines reactants, then measures the change in absorbance at a specific time interval over a specific period of time Enzymes Enzyme Concentration • As long as the substrate concentration exceeds the enzyme concentration, the velocity of the reaction is proportional to the enzyme concentration; ↑ enzyme, faster reaction pH • Enzymes are in its maximal activity at optimal pH (7.08.0) Temperature • ↑ temperature ↑ rate of reaction by increasing the movement of molecules • Every 10C ↑ - doubling of activity, until protein is denatured • At 65C = start of denaturation Cofactors • Activators and Enzymes Inhibitors • May act as reversible or irreversible inhibitors (permanent attachment to enzyme) • Competitive inhibitor o Physically binds to the active site of an enzyme and compete with the substrate for the active site; can be reversed by increasing substrate concentration • Noncompetitive inhibitor o Binds an enzyme at a place other than the active site and may be reversible or irreversible o Induces conformational change in the enzyme so that the substrate cannot bind • Uncompetitive Inhibitor o Inhibitor binds to the ES complex - cannot be reversed by adding more substrate Enzyme Measurement • Measure enzyme activity and reported in International Unit (IU) or less commonly in kat (katal). o 1 IU = 1 umol of substrate to produce/min o 1 kat = 1 mol of substrate/sec o 1 IU = 16.7 kat • Enzyme activity is reflected by measuring the small increase in product which is easier to do than measuring small decrease in large amount of substrate • Rate of Reaction - rate of disappearance of reactants or rate of appearance of products • Enzyme reaction may be coupled with another reaction where an indicator substance uses the products producing another product which is measured and reflects the amount of enzyme present Alkaline Phosphatase (ALP) • Non-specific enzyme capable of reacting with many different substrates • Optimal pH reaction is 9.0-10.0 • Activator: Mg2+ • Highest concentration in intestine, liver, bone, spleen, placenta, kidney • Isoenzymes o Placental - most heat stable of all normal ALP isoenzyme o Intestinal - Least Anodal o Liver - highest concentrations, most anodal (fastest) o Bone - most heat labile o Carcinoplacental isoenzyme include Regan, Nagao, and Kasahara (found in patients with malignancy) ▪ Regan is the most heat stable Clinical Significance • Evaluation of hepatobiliary and bone disorders • Cholestatis, hepatitis, and cirrhosis • Highest elevation in Paget's disease (osteitis deformans) • Other bone disorders: osteomalacia, rickets, hyperparathyroidism, and osteogenic sarcoma • Children, adolescents, pregnant women have higher values • Usually found in bile canaliculi - increased in blockages Enclonar, Kimberly / MLS 3A Methods • Reference Value: 30-90 U/L (30C) • 4 isoenzyme (electrophoresis migration) o Isoenzyme separation: Acrylamide gel, electrophoresis, chemical or heat (56C; 10mins) o Liver → Bone → Placenta → Intestinal • Heat Denaturation (Most heat stable to most heat labile) o Placenta → Intestinal → Liver → Bone • Substrate: Organic phosphates such as B-glyceroPO4 and p-nitrophenylPO4 • Other Methods: o Bodansky (B-glyceroPO4) o Shinowara-Jones-Reinhart (B-glyceroPO4) o King-Armstrong (phenylPO4) o Bessy-Lowry-Brock (p-nitrophenylPO4) o Bowers-McComb (p-nitrophenylPO4) - Most specific; pH 10.15 @405nm ▪ PNPP → p-nitrophenol + PO4- • Fewer amount in the kidney, pancreas and RBCs • Isoenzymes: Cytoplasmic & Mitochrondrial Clinical Significance • Mainly in evaluation of hepatocellular disorders and skeletal muscle o Found in hepatocytes • Increased in AMI within 6-8hrs; but not useful in diagnosis of AMI • Increased in viral hepatitis, cirrhosis, muscular dystrophies, and inflammatory conditions Methods • Reference Value: 5-35 U/L • Sources of error: Hemolysis can dramatically increase serum AST • Method: Karmen Method (uses malate dehydrogenase) - pH 7.5, 340nm Alanine Aminotransferase (ALT) Acid Phosphatase (ACP) • A hydrolase that catalyze the same type of reactions with ALP • Difference between ACP and ALP is the pH of reaction • Optimal pH approximately 5.0 • Found in prostate (highest), bone, liver, spleen, kidney, RBCs, and platelets • Isoenzymes: o RBC-ACP - inhibited by Copper o Prostate-ACP (pACP) - inactivated by Tartrate o Both differentiated using inactivation Clinical Significance • Aid in detection of prostatic carcinoma, particularly metastatic carcinoma • Not sensitive at early stage = prostate specific antigen is preferred • Proved useful in forensic CC: investigation of rape (seminal fluid-ACP activity; persists for 4 days) >50 IU/L Methods • Reference Value: o 2.5-11.7 U/L (Total ACP) o 0-3.5ng/mL (Prostatic ACP) • Very labile, add 5M acetate buffer or citrate tablet to preserve • Substrate: Organic phosphates such as B-glyceroPO4 and p-nitrophenylPO4 o Thymolphthalein monophosphate (most specific substrate for pACP) - for quantitative endpoint reactions (Roy and Hillman) o A-napthyl phosphate - for continuous monitoring • Other Methods o Gutman & Gutman (phenylPO4) o Shinowara (p-nitrophenylPO4) o Babson, Read, Philipps (a-napthylPO4) Aspartate Aminotransferase (AST) • • • • Belongs to the class of transferases Formation of glutamate + oxaloacetate Coenzyme: pyridoxal phosphate Older term: serum glutamic oxaloacetic transaminase (SGOT or GOT) - based on product • Transaminase • Highest concentrations are found in cardiac tissues, liver and skeletal muscle o Highest in cardiac tissues • A transferase with enzymatic activity similar to that of AST • Catalyzes transfer of amino group from alanine to aketoglutarate with the formation of glutamate and pyruvate • Coenzyme: pyridoxal phosphate • Older term: serum glutamic pyruvic transaminase (SGPT or GPT) • Highest concentration is in the liver which makes it more specific for liver disease than AST Clinical Significance • Evaluation of Hepatic disorders • Higher elevations found in hepatocellular disorders than extrahepatic or intrahepatic obstructive disorders • Acute inflammatory conditions of liver = ALT elevation is frequent than AST • Highest in viral hepatitis Methods • Reference Value: 7-45 U/L • Coupled Enzymatic Method o LD as the indicator enzyme o Change in absorbance at 340nm measured continuously is directly proportional to ALT activity o Optimal pH 7.3-7.8 • Reitmen Frankel o Alanine + a-ketoglutaric → pyruvic acid o Addition of DNPH (2,4-dihydrophenylhydrazine) to pyruvic acid to produce color AST/SGOT ALT/SGPT Substrate Aspartate aketoglutarate Alanine aketoglutarate End Product Oxaloacetic acid + glutamate Pyruvic acid + glutamate Location Heart, Liver, Skeletal Muscle Liver, Heart High Concentration Heart Liver Significance AMI Liver disease De Ritis Ratio Viral Etiology ↑ ALT ALT/AST >1 Non-viral ↑ AST ALT/AST <1 Enclonar, Kimberly / MLS 3A Amylase (Amy) • Belongs to the class of hydrolase • Smallest enzyme; catalyzes the breakdown of starch and glycogen • Activators: Calcium & Chloride • Major tissue source: acinar cells of pancreas and salivary glands • Lesser concentrations: Skeletal muscle, intestine, fallopian tube • Pancreatic AMS: amylopsin o Isoenzyme (P1, P2, P3) o P2 - commonly observed fraction • Salivary AMS: ptyalin o S1, S2, S3 o S1 and S2 - commonly observed fraction • Serum AMS: pancreatic in origin o MicroAMS ▪ Unbound, free; 50,000 dal. ▪ Found in urine o MacroAMS ▪ Bound to IgG, IgA; High MW ▪ Measured in serum Clinical Significance • Diagnosis of Acute pancreatitis - ↑ in P-type activity; predominantly P3 • ↑ level in salivary gland lesions, such as mumps and parotitis; perforated peptic ulcer, intestinal obstruction, cholecystitis, acute appendicitis • Earliest marker for acute pancreatitis o Found within 5-8 hrs (as early as 2 hrs) Methods • Reference Value o Serum: 28-100 U/L o Urine: 1-15 U/h • Saccharogenic o Measures the appearance of the product (glucose: Somogyi) o Somogyi: mL glucose released in 30mins at 37C • Iodometric/Amyloclastic o Measures the disappearance of the substrate (starch) o Decrease in dark-blue: iodine • Chromogenic o Measures dye released from breakdown of polysaccharide o Measures ↑ color intensity from the production of products coupled with a dye Kinetic • o Coupling of several enzyme systems to monitor amylase activity o Measures change of NAD to NADH at 340nm Lipase (Lps) • Hydrolyzes the ester linkages of fats to produce alcohols and fatty acids • Breaks down Tag into fatty acids and glycerol • Highest concentration in pancreas • Lesser amounts in stomach and small intestines • Isoenzymes: L1, L2, L3 o L2 - most clinically specific and sensitive Clinical Significance • Diagnosis of acute pancreatitis o Serum LPS activity increase 4-9hrs after attack o Concentration peak 24hrs o Decrease within 8-14 days • Most specific marker in diagnosing acute pancreatitis Methods • Reference Value: <38U/L • Cherry-Crandall (reference method) o Principle: Measures liberated fatty acids by titration after a 24hrs incubation o Substrate: 50% Olive oil (before); Triolein (more pure form of TAG) • Sigma-Tietz • Titration • Peroxidase-coupling - most common method used now Lactate Dehydrogenase (LDH) • Enzyme that catalyzes the interconversion of lactic acid and pyruvic acids • Highest concentration: heart, liver, skeletal muscle, kidneys, and RBCs • Lesser amounts in lungs, smooth muscle, and brain • Isoenzymes: o LD1 (anodic) o LD5 (cathodic) o LD1 & LD2 - heat stable o LD5 - most labile o 4 subunits (tetramer) o LD6 - alcohol dehydrogenase • Do not refrigerate! Isoenzyme Tissue Disorder LDH-1 (HHHH) Heart RBCs MI Hemolytic anemia LDH-2 (HHHM) Heart RBCs Megaloblastic anemia Acute renal infarct Hemolyzed specimen LDH-3 (HHMM) Lung Lymphocytes Spleen Pancreas Pulmonary embolism Extensive pulmonary pneumonia Lymphocytosis Acute pancreatitis Carcinoma LDH-4 (HMMM) Liver Hepatic injury or inflammation LDH-5 (MMMM) Skeletal muscle Skeletal muscle injury Clinical Significance • Increased levels in cardiac, hepatic, and skeletal muscle and renal disease • Least specific enzyme marker • Highest level is seen in pernicious anemia and hemolytic disorders • Increased in: Viral hepatitis, cirrhosis, AMI, some leukemias • Marked elevation: Acute lymphoblastic leukemia • Normal LD Isoenzyme concentration: o LD2>LD1>LD3>LD4>LD5 • If LD1>LD2 (Flipped ratio/pattern) - suggestive of AMI • Increased LD6 is seen in arteriosclerotic cardiovascular failure Methods • Reference Value: 125-220 U/L • Source of error: Hemolysis since RBS contain LD conc. approximately 100=150x found in serum • Interconversion of Lactic and Pyruvic acid using coenzyme NAD+ Enclonar, Kimberly / MLS 3A • Wacker Method (Forward/Direct Reaction) - pH 8.38.9 - measures pyruvate • Wrobleuski La Due (Reverse/Indirect Reaction) - pH 7.1-7.4 o Faster • Tanzer-Gilvarg (forward) o ↓ absorbance of NADH to NAD at 340nm (pH 9.0) o Uses additional pyruvate kinase and LDH Creatine Kinase (CK) • Other names: Creatine Phosphokinase (CPK); ATPcreatine-N-phosphotransferase • Extracted from the Greek word kreas • Identified in 1831 as a component of skeletal muscle • Nitrogenous organic acid which supplies energy to the cells and tissue • Activator: Mg2+ • Catalyzes the reversible reaction in the formation of ATP in tissues • Creatine + ATP → creatine phosphate + ADP • Found in heart, brain, skeletal muscles and others • CK-BB (CK1) o Brain type o Small quantity in tissue o Short half-life of 1-5hrs o High concentration in the CNS o "Blood-brain-barrier" o "Tumor-associated marker" • CK-MB (CK2) o Predominant in cardiac tissue (major source) o ≥6% increase of total CK is good indication of myocardial damage o Hybrid type o Level rise: 4-8hrs after MI o Peaks at: 12-24hrs o Declines: 48-72hrs o "time-frame" is crucial • CK-MM (CK3) o Muscle type o Major isoenzyme of the serum (94-100%) o Heart & skeletal muscle activity o Increased due to: ▪ Hypothyroidism, cardiac, and skeletal muscle injury ▪ Mild to strenuous activity ▪ Intramuscular injections • Macro-CK2 o Migrates between CK2 and CK3 • CK-Mi o Migrates slightly in opposite direction o Point cathodal to CK-MM Clinical Significance • Increased in: o AMI o Muscular dystrophy: Duchenne type (50-100x increase) o Hyperparathyroidism o Malignant hyperpyrexia o Reye's syndrome Methods • Reference value: o Male: 46-171 U/L o Female: 34-145 U/L o CK-MB: <5% total CK • Electrophoresis • Ion-exchange chromatography • Immunoassays o RIA o Immunoinhibition methods • Oliver-Rosalki (reverse) o ↑ in absorbance at 340nm (pH 6.8) o Uses additional hexokinase and G6PD Gamma-Glutamyltransferase (GGT) • Enzyme involved in the transfer of the y-glutamyl residue from y-glutamyl peptides to amino acids, H2O, and other small peptides • Most sensitive enzyme for all types of liver disease o Highest levels with obstructive disorders • Tested with ALP to confirm obstructive disorder • Marker for daily consumption of large amount of alcohol or drugs • Market for chronic alcoholism • Found in: Kidney, Brain, prostate, pancreas, liver Clinical Significance • Increased in: o Biliary tract obstruction o Patient taking warfarin, phenobarbital, and phenytoin (enzyme inducing drugs) o Chronic alcoholism o Pancreatitis, diabetes and MI Methods • Reference Value o Male: 6-55 U/L o Female: 5-38 U/L • Szaz Method o Substrate: gamma-glutamyl p-nitroanilide o Measured at 405-420nm o Principle: y-glutamyl residue is transferred to glycylglycine, releasing p-nitroaniline, a chromogenic product 5' Nucleotidase (5NT) • Responsible for catalyzing the hydrolysis of nucleoside-5'-phosphate esters • Significantly elevated in hepatobiliary disease • Secondary marker for obstructive jaundice • More sensitive to metastatic liver disease than ALP • Reference Value: 3-9U/L Pseudocholinesterase (ChE) • Secreted by the liver; representing synthetic function • Marker for insecticide/pesticide poisoning (organophosphate) • Important in the cleavage of succinylcholine • Only enzyme that is significant when ↓ • Method: Michael-Ellman o Substrate: Acetylcholine • Reference Value: 0.5-1.3 pH units (plasma) Enclonar, Kimberly / MLS 3A Reference Values Breast cancer RA ALP 30-90 U/L ACP 2.5-11.7 U/L (Total ACP) 0-3.5ng/mL (Prostatic ACP) AST 5-35 U/L ALT 7-45 U/L Amy Serum: 28-100 U/L Urine: 1-15 U/h CK MI Neuroleptic malignant syndrome Cysteine Cathepsins Premalignant lesions in colon, thyroid, brain, liver, breast, and prostate GGT Premalignant lesions in colon, thyroid, brain, liver, breast, and prostate Gelatinase-B Cardiovascular mortality Gastric cancer Vascular dementia RA Malignant gliomas Glycogen phosphorylaseBB MI G6P Gierke disease Hypoglycemia G6PD Gastric cancer Non-enzymatic MI Markers LDH Myoglobin • Major protein responsible for oxygen supply of striated muscle • Rapidly released into the bloodstream following muscle injury due to its abundance in cardiac muscle tissue and low molecular weight Pyroptosis Necrosis Breast cancer Leukocyte esterase Periprosthetic joint infection UTI Bacterial peritonitis Ascitic fluid infection Lipase Acute pancreatitis Lysozyme RA Tuberculous meningitis Tuberculous pericarditis Tartrate-resistant acid phosphatase Osteoarthritis Tartrate-resistant acid phosphatase-5b Giant cell tumor Bone metastases Lps <38 U/L LDH 125-220 U/L CK Male: 46-171 U/L Female: 34-145 U/L CK-MB: <5% total CK GGT Male: 6-55 U/L Female: 5-38 U/L 5NT 3-9 U/L ChE 0.5-1.3 pH units (plasma) Acute Myocardial Infarction Troponin • The troponin complex is a component of the thin filament of striated muscle linked to actin • 3 subunits: o Troponin I: an inhibitory subunit o Troponin T: tropomyosin-binding subunit o Troponin C: calcium - binding subunit Enzyme Disorder/Disease ACP Malaria Prostatic carcinoma ALT Hepatocellular damage Hepatitis B and C ALP Chronic kidney disease Paget Disease or rickets/osteomalacia Type II diabetes Obstructed liver Amylase Pancreatitis MI AST Hepatic disease Dental disorder Liver fibrosis Butyrylcholinesterase Schizophrenia Alzheimer's disease Parkinson's disease Cathepsin-D Renal cell carcinoma Dental disorder Case Study 1 • Age: 40 • Chief complaints: Fever, vomiting, indigestion, diarrhea, pain on the right side radiating to the lower back up to the shoulders • Lab results: o WBC ↑ o Lipase N o ALT, AST, ALP, Total Bilirubin ↑ • Disease: Hepatitis Case Study 2 • Age: 39 • Chief complaints: The patient was brought to the emergency department via ambulance due to decreased level of consciousness, nausea, and vomiting • Lab results: o ALT, AST normal o Total Bilirubin, Lipase ↑ o Amylase ↑↑ Disease: Acute pancreatitis • Enclonar, Kimberly / MLS 3A Myoglobin Trop T Trop I CK-MB AST LD Elevation after 2-4 hrs (1st chest pain (MI) nonenzyme/protein to rise) 3-4 hrs 3-12 hrs 4-8 hrs (1st enzyme to rise) 6-8 hrs 8-10 hrs Peak Activity 6-10 hrs 48 hrs 12-24 hrs 12-24 hrs 24-48 hrs 72 hrs Duration of elevation 2-5 days 2-5 days 5-10 days 2-3 days 4 days 10 days Sensitivity (increase immediately)/ Specificity Sensitive but not specific More sensitive and specific than CK-MB More sensitive and specific than CK-MB Not entirely specific MI Not sensitive, not specific Insensitive, nonspecific Usefulness Negative predictive marker More sensitive and specific than CK-MB More sensitive and specific than CK-MB Used to be the "gold standard" Used to detect infarction >3 days prior to testing Used to detect infarctions occurred >5 days prior to testing Eliminates need for LD isoenzyme Eliminates need for LD isoenzyme Negative results within first few hours after chest pain; rule out MI Popularity is declining due to newer tests Gold standard Methods Latex agglutination ELISA Immunonephelometry Fluoroimmunoassay ELISA ELISA E'phoresis RIA Immunoinhibition E'phoresis Immunoinhibition Troponin and CK-MB - recommended for MI 2 samples, maximum 3 • 1st sample - at presentation • 2nd sample - after 6-9 hrs • 3rd sample - after 12-24 hrs MI according to Bishop (M Trop CAL <3) Myoglobin Trop T Trop I CK-MB AST LDH Onset 2-3 hrs - - 4-8 hrs 6-8 hrs 12-24 hrs Peak 8-12 hrs - - 12-24 hrs 24 hrs 48-72 hrs Duration of Elevation 18-30 hrs - - 48-72 hrs 5 days 10 days Disease Condition Lab Findings Alcohol Fatty Liver Slight elevations of ALT, AST, GGT, with fatty infiltrates Alcoholic Hepatitis Moderate elevations of ALT, AST, GGT, ↑ Total Bilirubin, ↑ PT, ↓ Protein Alcoholic Cirrhosis Elevated ALT, AST, GGT, with gastrointestinal bleeding