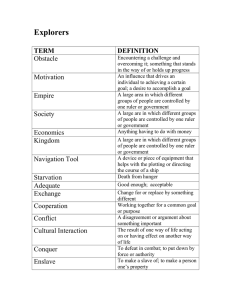
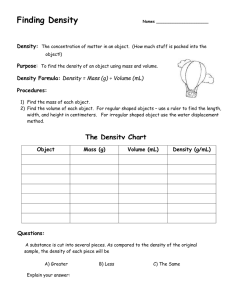
Lab Report 1 Meena, Iyonna, Porter, and Daniel Afolabi 21. February, 2024 Introduction The concept being investigated is density, which is defined as the mass per unit volume of a substance. Density can be used to determine if two objects are made of the same material because objects made of the same material will have the same density. Therefore, density can help answer the guiding question of whether the two objects in this investigation are made of the same material. Methods To determine the density, the mass and volume of each object needs to be measured. A balance was used to find the mass of each object. Two methods were used to find the volume water displacement and calculations using ruler measurements. The water displacement method involved filling a graduated cylinder with a known with 50mL of water, submerging the object to find the new total volume, and taking the difference between the two volumes. This method is very accurate. The ruler method involved taking length and diameter measurements of the objects with a ruler and calculating the volume using the equation for volume of a cylinder (V = πr2L). We used a spill can to measure the volume of water displaced by each object. This helped to reduce error by minimizing the amount of water that was spilled. We also took multiple measurements of each object and calculated the average value. This helped to improve the reliability of our results and reduce random errors. Results and Discussion The data for this experiment is shown in the following table: Mass (g) Object A (silver) 13.565 Volume (displaceme nt, mL) 5.1 Object B (black) 7.948 5.1 Volume (ruler, cm³) 5.24 Density (displaceme nt, g/mL) 2.7 Density (ruler, g/cm³) 2.89 5.63 1.6 1.41 Volume of a cylinder = πr2L Density = Mass / Volume The average density of Object A (silver) was 2.7 g/mL using water displacement and 2.89 g/mL using ruler measurements. For Object B (black), the densities were 1.6 g/mL and 1.41 g/mL for the two methods, respectively. The water displacement method is more accurate, so those density values carry more weight. The density of Object A is higher than Object B, indicating they are made of different materials. Therefore, my claim is that Object A and B are not made of the same material. The density values support this claim because if the objects were the same material, their densities would be equal. The water displacement densities differ by 1.1 g/mL, which is a significant difference, providing strong evidence that they are different materials. The ruler measurements also show different densities, although the values are less reliable because of systematic errors that may happen during imprecise measurements with a ruler. There is some variation between trials, indicating random error. Overall, the density data provides sufficient evidence to confidently state the objects are not the same material. Conclusion In conclusion, based on the density analysis, Object A (silver) and Object B (black) do not have the same density and are therefore made of different materials. My claim is that the two objects are not made of the same material, which is supported by the density evidence calculated in this investigation.