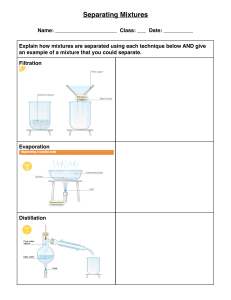
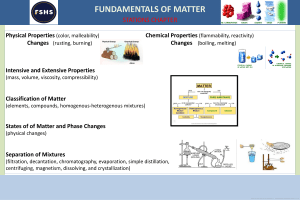
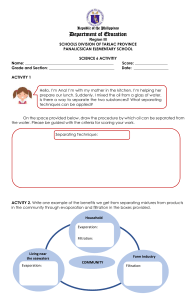
Lesson Plan parts based on the guidelines from D.O. no. 42 s. 2016 V School: Teacher: Date: I. Lupok Central Elementary School Jovita P. Holanda Grade Level: Learning Area: Quarter: 6 Science 1 Objective: 1. Content Standards: The learners demonstrate understanding of different types of mixtures and their characteristics. 2. Performance Standards: The learners should be able to prepare beneficial and useful mixtures such as drinks, food, and herbal medicines. 3. Most Essential Learning Competency: I. Enumerate techniques in separating mixtures. S6MT-ld-f-2 Identify the processes and tools involved in separating homogeneous mixture. o Evaporation o Distillation o Rectification o Chromatography II. Content: Separating Mixtures (Homogeneous) III. Learning Resources: References I. Teacher’s Guide Science Beyond Borders pp.11-13 II. Textbook Science Beyond Borders pp. 21-25 The New Science Links 6 pp. 90-91 Other learning Resources- PowerPoint Presentation, video IV. Procedure: 1. Reviewing past lesson/Presenting the new lesson I. Review homogeneous mixtures. II. New lesson: Separating Homogeneous Mixtures: Evaporation, Distillation, Rectification, chromatography III. New words: Evaporation Collected Stainless` Centrifugation Molasses Fermented Rectification Nitrogen Essential Distillation evaporate separation complicated crystals machine condensation community techniques 2. Establishing a purpose for the lesson I. Pose this question to the learners: A. What are the techniques of separating homogeneous mixtures? B. What components are separated in the mentioned techniques? 3. Discussing new concepts and practicing new skill no.1 I. Discussion: There are ways in separating homogeneous mixtures. A. Evaporation- a technique used in separating soluble solids from a liquid. Example: producing salt through evaporation. B. Distillation – a process of separating liquids from solutions. This process involves distillation and condensation. Example: producing distilled water. C. Show videos showing evaporation and distillation. II. Explain. 1. What is evaporation? 2. What is separated in evaporation? 3. What is distillation? 4. What is separated in distillation? 5. What processes is involved in distillation? 4. Discussing new concepts and practicing new skill no. 2 I. DiscussionA. Rectification – the process for separating mixed gases. Air is turned into liquid first. The resulting liquid is then heated. Each component of the liquefied air evaporates and turns into gas at different temperatures. Example: garnering oxygen from the atmosphere. B. Chromatography – is a technique that separates dissolved substances that have different colours. This technique works in separating dyes. Example: used in pharmaceutical industries to separate and purify drugs. C. Show a video of rectification and chromatography. II. Explain: 1. What is 2. What is 3. What is 4. What is rectification? separated in rectification? chromatography? separated in chromatography? 5. Developing mastery Let the learners read the Science Concepts learned. Multiple choice test Read each item carefully. Choose the letter of the best answer from the choices. 1. When separating the components of a solution, what change is required on one of its components? a) A change in size. b) A change in shape. c) A change in phase. d) A changed in smell. 2. What process is appropriate to get drinking water from sea water? a) Condensation b) Evaporation c) Rectification d) Evaporation and condensation 3. What processes are involved in distillation? a) Separation and evaporation b) Evaporation and condensation c) Centrifugation and condensation d) Filtration and evaporation 4. How do you separate into its different components the mixture of miscible liquids? a) Centrifugation b) Distillation c) Evaporation d) rectification 5. When separating alcohol and water, which is boiled off and why? a) Alcohol, because it has a lower boiling point. b) Alcohol, because it floats on top of water. c) Water, because it has a higher boiling point. d) water, because it is the solvent. 6. At what temperature should a mixture be heated in order to separate two miscible liquids with boiling point at 50˚C and 80˚C? a. Just below 50˚C b. 50˚C or a few degrees higher. c. 80˚C or a few degrees lower. d. Just a bit higher than 80˚C. 7. Why is there a need for rectification of air? a. So that specific gases can be for different purposes. b. So that gases become liquids. c. So that the air is cooled. d. So that air is heated. 8. Why is distillation an effective way of separating alcohol and water? a. Because water and alcohol have the same boiling point. b. Because water and alcohol have different boiling points. c. Because water and alcohol have the same colour. d. Because water and alcohol have the same solubility. 9. Why do some people prefer drinking distilled water than tapped water? a. Distilled water has gases removed from it. b. Distilled water has additional nutrients. c. Distilled water has a sweeter taste than tapped water. d. Distilled water has impurities removed from it. 10. What do you call the liquid that evaporates, then condenses via a long tube into a collecting vessel during distillation? a. Solute b. Solvent c. Residue d. Distillate b. Finding practical applications of concepts and skills in daily living A. Pupils wanted to separate a sugar solution from the oil that they put in a beaker/glass. What method or technique of separation would they use? B. Which process is appropriate in purifying water? a) Centrifugation b) Condensation c) Distillation d) filtration a. Making generalizations/abstraction about the lesson A. What are the techniques of separating homogeneous mixtures? B. What components are separated in the mentioned techniques? b. Evaluating Learning: 1. Enumerate the techniques in separating homogeneous mixtures. 1. ______ 2. ______ 3. _______ 4. _______ 2. What components are separated in the mentioned techniques? Process or technique in separating homogeneous mixture 1. Evaporation 2. Distillation 3. Chromatography 4. Rectification Components being separated c. Additional activities for application or remediation/Assignment Make some research on the different techniques of separating heterogeneous mixtures. Prepared by: JOVITA P. HOLANDA MT- 2 Observers: ___________________________ _______________________________